
CASE SUMMARY
BACKGROUND: A 49-year-old female was referred to our cathlab for an extensive anterior STEMI complicated by cardiogenic shock.
INVESTIGATION: Coronary angiography.
DIAGNOSIS: Left coronary angiography revealed occlusion of the left main stem with evidence of massive intracoronary thrombus.
MANAGEMENT: Manual thrombectomy followed by super-selective adenosine injection by thrombus aspiration catheter combined with an intracoronary bolus of abciximab was performed. A drug-eluting stent was implanted in the left main and proximal left descending artery.
KEYWORDS: cardiogenic shock, crushed ticagrelor, hyperhomocysteinaemia, intra-aortic balloon pumping, STEMI
PRESENTATION OF THE CASE
A previously healthy 49-year-old woman without any prior medical history was referred to our hospital for primary percutaneous intervention, 77 minutes after the onset of acute chest pain. ECG by emergency medical system (EMS) revealed an extensive anterior ST-segment elevation. Intravenous aspirin 250 mg and 5,000 UI of unfractionated heparin were administered by EMS. On arrival at the cathlab the patient was in cardiogenic shock (SBP <60 mmHg). Due to the absence of both radial and femoral pulses, the right femoral artery was accessed under fluoroscopic guidance. Meanwhile, the patient was intubated and intravenous administration of dopamine and adrenaline was initiated. This resulted in a modest increase of SBP (80 mmHg). In order to make a speedy appraisal of the patient’s conditions, the decision was taken to proceed immediately with coronary angiography. Left coronary angiography revealed occlusion of the left main (LM) stem with evidence of massive intracoronary thrombus. Right coronary angiography demonstrated absence of both atherosclerotic lesions and collaterals towards the left coronary artery (LCA) (Figure 1A, Figure 1B).
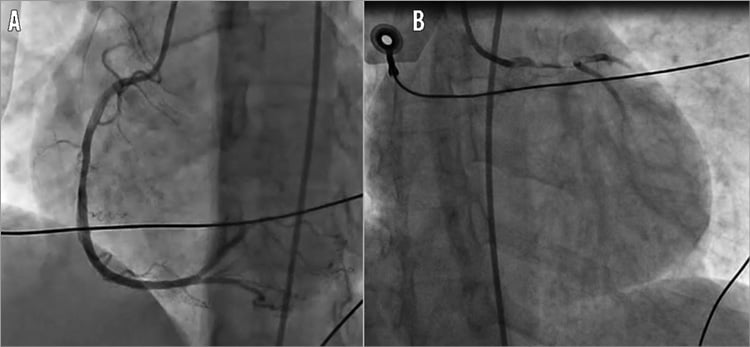
Figure 1. Angiography on admission to cathlab. A) Right coronary angiography showed absence of both atherosclerotic lesions and collaterals towards the left coronary artery. B) Left coronary angiography revealed the occlusion of the left main stem with evidence of massive intracoronary thrombus.
Subsequently, the operators tried to restore coronary flow. The initial approach included attempts to advance guidewires into the left descending artery (LDA) and the left circumflex artery (LCX). After successfully wiring two main vessels, manual thrombectomy using the Eliminate™ aspiration catheter (Terumo Europe NV, Leuven, Belgium) was performed. Despite multiple runs of thrombectomy, a large burden of thrombus remained on the angiographic images (Figure 2). Super-selective adenosine injection by thrombus aspiration catheter combined with an intracoronary bolus of abciximab provided the partial restoration of coronary flow. A direct drug-eluting stent implantation on the left main and proximal LDA tract resolved the acute scenario (Figure 3A). Angiographic control revealed the patency of both LDA and LCX with a residual thrombus burden in the LCX ostium (Figure 3B). After restoration of TIMI flow grade 3 in both the LDA and the LCX, an intra-aortic balloon pump (IABP) was positioned. Our decision-making process faced two main issues: the best pharmacological therapy and the optimal interventional strategy for this patient.
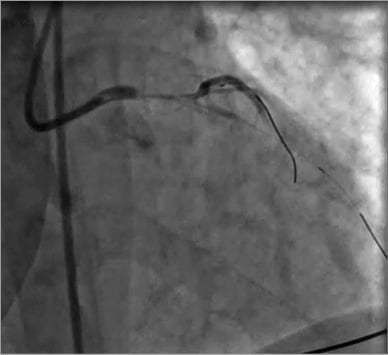
Figure 2. Angiography after manual thrombectomy. After manual thrombectomy a large burden of thrombus remained on the angiographic images.
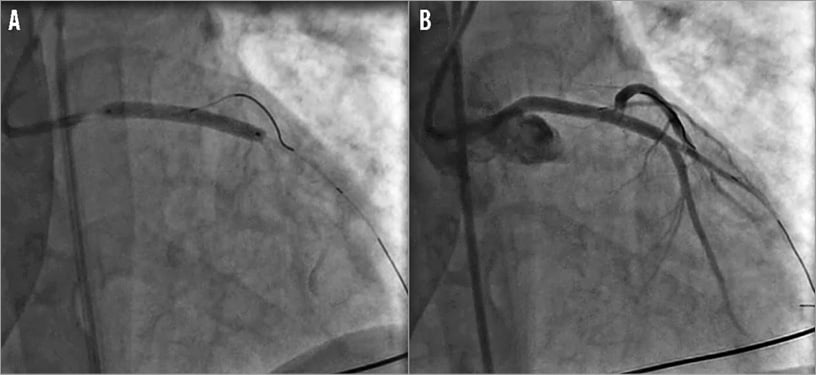
Figure 3. PCI of the left main - LDA tract. A) Direct drug-eluting stent implantation on the left main and proximal LDA. B) Angiographic control showed the patency of both the LDA and the LCX with a residual thrombus burden in the LCX ostium.
How would I treat?
THE INVITED EXPERTS’ OPINION

Marchese et al present the case of a 49-year-old female patient in cardiogenic shock complicated by an anterior ST-elevation myocardial infarction (STEMI). Our treatment strategy would be based on current evidence on the management of patients with cardiogenic shock1.
1. First and foremost it is important to emphasise that the patient should be transferred directly to the catheterisation laboratory and emergency revascularisation should not be further delayed. Early revascularisation, as shown in the SHOCK trial, is the most important therapeutic intervention in cardiogenic shock complicating acute myocardial infarction2.
2. In the setting of haemodynamic instability requiring catecholamines and inotropes we would administer norepinephrine and dobutamine. Based on the findings of the SOAP II trial, including 1,679 haemodynamically unstable patients of whom 280 were in cardiogenic shock, we would anticipate fewer arrhythmic events with this combination in comparison to dopamine3.
3. Following a diagnosis of left main occlusion one might think about coronary artery bypass grafting (CABG). In current ESC guidelines both CABG and percutaneous coronary intervention (PCI) in left main stenosis and a SYNTAX score <22 are recommended equally with a class IB indication4. However, in the setting of STEMI and cardiogenic shock, restoration of myocardial perfusion as quickly as possible is crucial. Thus, we would definitively go for the faster interventional approach. Further, fewer than 5% of the patients in cardiogenic shock undergo immediate CABG and to date there are no data indicative of a benefit of CABG over PCI in the setting of cardiogenic shock.
4. Despite the negative results of the TASTE and TOTAL trials, where routine thrombus aspiration before PCI as compared with PCI alone did not reduce clinical events among patients with STEMI, we would perform manual thrombectomy5,6. Hypothetically, the subgroup of patients with high thrombus burden could profit from this intervention.
5. We would then go ahead with the procedure using drug-eluting stents. As the thrombus is present in both the left anterior descending as well as in the circumflex, we would plan a two-stent strategy. As few systematic data support a specific stent technique in left main stenoses, we would go for a simple and fast T-stenting.
6. In case of slow-flow or persistent thrombotic material, we would administer a bolus of a glycoprotein IIb/IIIa inhibitor with a subsequent 12-hr intravenous infusion. The route of bolus, either intravenously or intracoronary, does not affect clinical outcome based on the AIDA-STEMI trial, so we would be indifferent about that. Further, one can argue in favour of a liberal use of glycoprotein IIb/IIIa inhibitors, as administration and resorption of oral platelet inhibitors in patients with cardiogenic shock could potentially be delayed.
7. Based on data of the MOJITO study, we would administer crushed tablets of ticagrelor with a loading dose of 180 mg via a gastric tube as soon as possible.
8. Finally, if cardiogenic shock was refractory to standard treatment following rapid revascularisation as well as optimal fluid and catecholamine management, we would implant an active left ventricular assist device or an extracorporeal life support system. Based on the IABP-SHOCK II trial failing to demonstrate an impact of intra-aortic balloon counterpulsation on clinical outcome, we would refrain from using this device.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

At this critical moment “time is myocardium” truly reflects our top priority. Marchese and colleagues are to be highly commended for their excellent work of successful intracoronary injection of abciximab, thrombus aspiration, and subsequent coronary stenting, which promptly restored TIMI grade 3 anterograde coronary perfusion in both left anterior descending and left circumflex artery territories. Obviously, this 49-year-old lady’s final outcome depends on the direction of her fluctuating clinical progress. If she is lucky enough, her condition may be temporarily stabilised after the application of intra-aortic balloon pumping (IABP) and all of the above-mentioned interventions with supplementary inotropic and ventilator support8. However, it is much more likely that her haemodynamic condition will continue to deteriorate due to acute massive myocardial infarction and persistent cardiogenic shock. In fact, the in-hospital mortality rate in such a rare subset of patients (i.e., with acute complete left main coronary occlusion and cardiogenic shock) may be greater than 60-70%9. Moreover, the chance of consequential development of multiple organ dysfunction in this group of patients is almost inevitable. Thus, quick establishment of efficient therapy with extracorporeal life support appears essential at this stage9-16.
The percutaneous ventricular assist device (VAD) may be considered as the first-line treatment choice under such life-threatening circumstances. Commonly accessible therapeutic options include use of the Impella® device (Abiomed, Danvers, MA, USA) and the application of venoarterial extracorporeal membrane oxygenation (ECMO). Indeed, some recent case reports10,11,13 and registries9,12 have highlighted the positive impact of rapid activation of such services on reducing mortality in this drastically ill patient population. In some patients with refractory cardiogenic shock or multi-organ failure, treatment with VAD may serve as a “bridge to heart transplantation”11 or a “bridge to decision”14. Clearly, without the collective efforts of every member of a multidisciplinary Heart Team, involving cardiologists, cardiac surgeons, anaesthesiologists and intensive care specialists, hospital survival for these patients can hardly be improved.
A small Canadian registry12 reported “the lowest mortality rate” of 38% in eight patients with acute complete left main coronary occlusion and cardiogenic shock, which may be of particular interest and relevant to the current case. Firstly, similar to this 49-year-old lady, all eight Canadian patients had a right-dominant coronary circulation, with a patent right coronary artery, although no right-to-left collateral vessels were visualised during coronary angiography12. In terms of interventional and pharmacological therapies, successful PCI of the left main culprit was achieved in all eight patients and there was universal use of glycoprotein IIb/IIIa inhibitors. Meanwhile, IABP was used in seven patients, ECMO or VAD in four patients, and post-PCI CABG was required in two patients12. Logically, the combined utilisation of these aggressive therapeutic modalities was believed to contribute to the improved overall survival2,15. Although such strategies have yet to be further validated15,16, they are certainly worth including in the “to-do list” for this 49-year-old lady.
Last but not least, few would dispute that better clinical results can only be achieved when the Heart Team is able to start these interventions “as early as possible”.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
A 49-year-old female was referred to our cathlab for an extensive anterior STEMI complicated by cardiogenic shock. This is a complex clinical scenario in which surgical revascularisation should have been considered. Both European and American guidelines suggest emergency revascularisation with either PCI or CABG (Class IA) in patients with cardiogenic shock due to pump failure after STEMI17,18. Nevertheless, due to the lack of randomised trials in this setting, uncertainty surrounds the optimal revascularisation strategy for patients with STEMI and unprotected left main (ULMCA) occlusion19. Due to the critically ill nature of these patients and to selection bias of the two groups’ baseline characteristics, there are no randomised trials comparing PCI and CABG in this setting. PCI is the preferred alternative option in appropriate patient groups with cardiogenic shock, favourable anatomy and low procedural risk, given the very high surgical mortality. The higher risk of target vessel revascularisation is an acceptable trade-off given the rapid reperfusion and potential reversal of haemodynamic instability achievable with a timely PCI. In order to achieve an immediate coronary flow restoration, we performed the LM-LDA PCI. Even though the angiographic control revealed a residual thrombus burden in the LCX ostium, we decided to continue with a conservative approach (Moving image 1). Considering the massive thrombus burden, we carefully balanced the technical challenges of a two-stent left main procedure. Our revascularisation strategy was in line with published data which reported only 8% of ULMCA lesions treated with complex bifurcation techniques20.
The decision-making process faced another important issue: the pharmacological therapy. Intravenous antithrombotic drugs such as GP IIb/IIIa inhibitors remain a favourable option in cardiac shock conditions because their treatment efficacy does not require any prior active absorption or in vivo bioactivation. In addition, the intracoronary use of abciximab proved its efficacy in the context of a large burden of thrombus but at the expense of increased bleeding21. Our patient presented several clinical and procedural factors contributing to a higher bleeding risk during IABP support: female gender, body surface area <1.65 m2, and a double transfemoral approach22. We carefully evaluated the best efficacy/safety balance for GP IIb/IIIa inhibitors and decided on intracoronary followed by intravenous abciximab administration in a high thrombus burden scenario.
Of note, absorption and effectiveness of oral P2Y12 inhibitors (P2Y12i) are significantly impaired in STEMI patients due to insufficient gastroduodenal motility and liver hypoperfusion. These mechanisms, combined with catecholamine and opioid use, are even more relevant in the subset of haemodynamically complicated STEMI patients23. Conversely, the higher platelet reactivity in STEMI patients seems to affect seriously the P2Y12i onset of action24. Prasugrel and ticagrelor have proved to be more rapid and effective as compared to clopidogrel but cardiogenic shock patients are an understudied cohort, excluded from prior large-scale trials comparing new P2Y12i with clopidogrel25,26. Ticagrelor, as an active drug independent from any in vivo bioactivation steps, could have a potential advantage over both pro-drugs clopidogrel and prasugrel. Moreover, in STEMI patients, crushed ticagrelor tablet administration, via a nasogastric tube, is feasible and seems to provide faster platelet inhibition than standard integral tablets27. Nevertheless, these patients are also excluded from the ongoing MOJITO clinical trial. Hence, in the absence of solid evidence on potent platelet inhibition in this subset, we hypothesised that 180 mg ticagrelor crushed pills via a nasogastric tube might have a theoretical advantage from a pharmacological perspective.
At the end of the procedure in the cathlab, an IABP was placed in position. The role of IABP to provide haemodynamic support in patients undergoing high-risk PCI is largely debated. Owing to the difficulty of performing randomised trials in this setting, there is somewhat conflicting evidence with respect to the benefit of IABP in cardiogenic shock patients28. IABP-related complications, ranging between 15% and 30%, might explain the lack of beneficial effect in terms of mortality22. Nevertheless, by reducing afterload and myocardial oxygen consumption, the use of IABP is particularly indicated in case of left ventricular dysfunction complicating an ischaemic insult, with a Class IIB ESC guidelines recommendation17.
In line with our decision, the IABP-SHOCK II trial showed no mortality benefit in the subgroup of patients in whom the IABP was positioned before the start of revascularisation, as compared with those in whom it was inserted after revascularisation.
Two days after the procedure, the IABP was removed, and a transthoracic echocardiogram demonstrated an improvement of ejection fraction (50%) with left ventricular apex akinesia.
Laboratory examination showed a significant troponin concentration rise (peak 155 ng/ml) with moderate hyperhomocysteinaemia (21.3 mmol/l) and normal thrombophilic profile. Regardless of other risk factors, a mild to moderate increase in homocysteine levels has been found to be associated with a higher risk of atherosclerotic vascular disease29. Even though the association between acute arterial thrombosis and hyperhomocysteinaemia has rarely been described, several mechanisms might explain this phenomenon: increase of tissue factor expression and factor V activity, suppression of thrombomodulin activity, decreased fibrinolysis30. This finding provides a possible explanation for acute coronary artery thrombosis occurring in a 49-year-old woman with no risk factors and strongly supports the diagnosis of a hypercoagulable state as the underlying cause of the acute event. The patient was eventually discharged on dual antiplatelet therapy and on folic acid and vitamin B12 supplementation. One-month angiographic follow-up revealed TIMI 3 flow into the LDA and LCX (Moving image 2).
Conclusions
This case showed that primary PCI is a viable treatment modality for ULMCA culprit lesions, especially in those patients with cardiogenic shock at presentation and a massive thrombus burden scenario. Further studies and randomised trials, even though difficult to conduct, are needed to elucidate the optimal interventional and pharmacological strategies with respect to stenting technique and antiplatelet therapy in this patient setting. Beyond the guidelines, an accurate case-by-case approach should be considered on the basis of ULMCA anatomy and patient haemodynamics. Moreover, we suggest that prothrombotic factors should be carefully evaluated in patients with premature coronary artery disease and absence of other risk factors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Final angiographic control.
Moving image 2. One-month angiographic follow-up.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Final angiographic control.
Moving image 2. One-month angiographic follow-up.

