CASE SUMMARY
BACKGROUND: A 72-year-old woman with non-ST elevation acute coronary syndrome, treated with fondaparinux.
INVESTIGATION: The patient was treated after recurrent angina with coronary angioplasty and stenting of the left anterior descending artery (LAD). The procedure was complicated by “swinging” left main (LM) thrombosis.
diagnosis: Catheter-related LM thrombosis after fondaparinux administration.
TREATMENT: Urgent LM angioplasty with culotte stenting of LM, LAD, and left circumflex coronary artery (LCx), and thrombus aspiration.
KEYWORDS: Fondaparinux, coronary angioplasty, drug-eluting stent, left main coronary occlusion, catheter-related coronary thrombosis.
PRESENTATION OF THE CASE
A 72-year-old woman was referred for episodes of chest pain radiating to left arm and associated with dyspnoea and sweating that had continued over a couple of days. She was obese, diabetic and hypertensive, however without history of heart disease; she was receiving therapy with insulin and irbesartan.
Systolic arterial blood pressure at admission was 170/100 mmHg, and physical examination showed tachycardia and basal bilateral rales. ECG showed sinus rhythm (90 bpm) and mild anterior ST-elevation in V1-V3 and negative T waves in V1-V6 leads. Chest radiography showed signs of mild pulmonary congestion, with a mildly enlarged transverse cardiac diameter. Cholesterol, and creatinine levels were normal; troponin I was 3.89 ng/ml.
The patient was admitted to the coronary care unit, treated with diuretics, aspirin, clopidogrel, atorvastatin, levosimendan and fondaparinux, and scheduled for a coronary angiography after haemodynamic stabilisation. Echocardiography showed a mild systolic dysfunction (45% left ventricular ejection fraction), with apical akinesis.
Seven days after admission, when in stable haemodynamic conditions, therapy with fondaparinux was shifted to enoxaparin and the patient underwent coronary angiography. At cathlab examination, a severe proximal left anterior descending (LAD) coronary stenosis was found (Figure 1A, Moving image 1A); no other remarkable narrowing was detectable elsewhere. LAD stenosis was successfully treated with PCI and sirolimus-eluting stenting (SES) (Figure 1B, Moving image 1B).
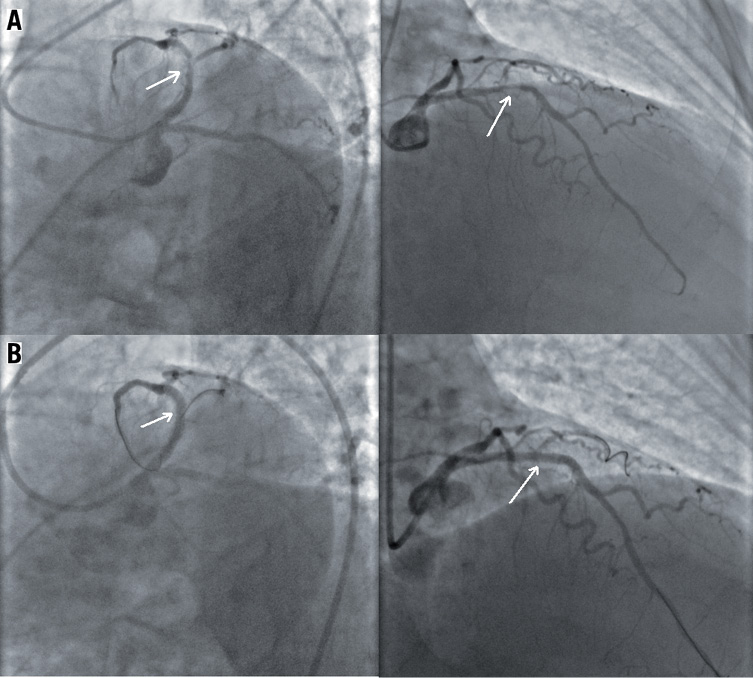
Figure 1. A) Severe proximal left anterior descending coronary stenosis before; B) After angioplasty with drug-eluting stent.
A few minutes after stent deployment, signs of massive thrombosis were suddenly detectable in the LM coronary artery, extending into the proximal left circumflex coronary artery (LCx) (Figure 2, Moving image 2). ECG immediately showed severe anterior ST-elevation with precipitating systolic blood pressure (60-40 mmHg) values and onset of ventricular fibrillation, promptly treated with defibrillator shock.
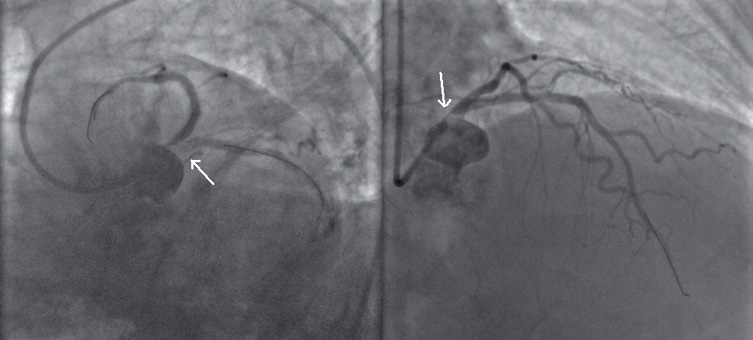
Figure 2. Massive thrombosis of left main coronary artery, extending into proximal left circumflex coronary artery.
How would I treat?
The invited expert’s opinion
Two different aspects of this case have to be discussed.
First, the best treatment of thrombus formation during percutaneous coronary intervention (PCI) is to prevent it from occurring at all. In this regard, the therapeutic approach adopted for this patient is debatable. The patient is a hypertensive, obese, diabetic patient presenting with high-risk non-ST elevation myocardial infarction (troponin elevation, ST-T wave changes, diabetes, impaired left ventricular function), complicated with heart failure. In such a case, the recommendation is to give anticoagulation, dual antiplatelet therapy, beta blockers, statins, angiotensin converting enzyme (ACE) inhibitors plus symptomatic treatment of hypertension and heart failure, and to take the patient to the cathlab within the first 24 hours for revascularisation (PCI or coronary artery bypass graft [CABG]), if technically feasible1,2. Glycoprotein IIb / IIIa inhibitors are rarely used for this indication despite being quite strongly recommended (recommendation likely to change in the next set of guidelines for the management of non-ST-segment elevation acute coronary syndromes, given the results of the EARLY ACS trial). In this report, the authors have chosen fondaparinux as the anticoagulant. If PCI is undertaken using fondaparinux, it must be carried out with unfractionated heparin (UFH), as well as fondaparinux, given as a single bolus at standard dose when starting the PCI procedure1,2. Indeed, the level of anticoagulation achieved with fondaparinux at a standard 2.5 mg subcutaneous daily dose is modest, as shown by the lower levels of anti-Xa activity and inhibition of thrombin generation achieved with fondaparinux as compared to enoxaparin at the doses used in the OASIS-53 trial. This low level of anticoagulation achieved with fondaparinux is sufficient to protect against recurrent ischaemic events prior to revascularisation4. It is clearly not sufficient to protect fully against thrombus formation during PCI. For this reason, UFH, or alternatively bivalirudin, is needed as well as fondaparinux in case of PCI5. In addition, in this particular case, switching from fondaparinux to enoxaparin before angiography/PCI is illogical, since catheter thrombus during PCI was observed with both drugs (although significantly more frequently with fondaparinux) in Oasis-54. Moreover, the bioavailability of subcutaneous drugs in obese patients is thought to be suboptimal. Furthermore, strictly speaking, thrombus formation during PCI did not occur with fondaparinux on board since the authors indicate that enoxaparin was substituted for fondaparinux before diagnostic angiography/PCI.
Secondly, as regards treatment of such an acute complication when it occurs, the best solution is thrombus aspiration after full anticoagulation has been achieved with a standard dose of unfractionated heparin or bivalirudin. Bail-out glycoprotein IIb/IIIa inhibitors, such as abciximab, can be useful. It may be necessary to crush the thrombus with repeat, low-pressure balloon inflation, with or without a distal protection device, if thrombus aspiration does not fully restore patency. Stenting should be avoided.
Conflict of interest statement
The author has no conflict of interest to declare.
How would I treat?
The invited expert’s opinion
This is a case of intra-procedural thrombus formation in the left main coronary artery. Unknown procedural factors, such as inadequate dosing of antithrombotics, chemical irritation of any interventional device, or coronary dissection might have caused the complication. Regarding the treatment strategy, wiring into the circumflex should be performed quickly for luminal patency and subsequent treatment. After wiring, aspiration thrombectomy using an aspiration catheter or a small (4 Fr or 5 Fr) guiding catheter with the mother-child technique should be performed in addition to the provisional use of a glycoprotein IIb/IIIa inhibitor. Aspiration should be carried out very gently to prevent thrombus embolism to the distal coronary artery or aorta. After aspiration, stenting may be required if dissection is detected in the lesion by angiography and/or IVUS examination. In such a case, the stenting technique is determined by intravascular ultrasound (IVUS) findings. If there is no dissection or flow-limiting obstruction, the lesion may not need further treatment. Instead, anticoagulation treatment with or without glycoprotein IIb/IIIa inhibitors, as well as and dual antiplatelet therapy should be administered.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF CASE
After an incomplete thrombus aspiration, a SES was placed into the LM and LCx, with near-optimal vessels patency. However, immediately after LM and proximal LCx stenting, angiography showed a complete occlusion of the proximal LAD (Figure 3A, Moving image 3A). Thrombus aspiration through stent struts was now impossible, so a LM SES stent was crossed with a guidewire and proximal LAD stenting was performed with an everolimus-eluting stent (EES) (culotte stenting) (Figure 3B, Moving image 3B). The procedure was completed with a final kissing balloon dilatation (Figure 4A and Figure 4B, Moving image 4A) with a complete patency of LM, LAD and LCx (Figure 4C, Moving image 4B) and intracoronary abciximab administration. The patient’s clinical conditions immediately improved and remained stable during the entire in-hospital stay.
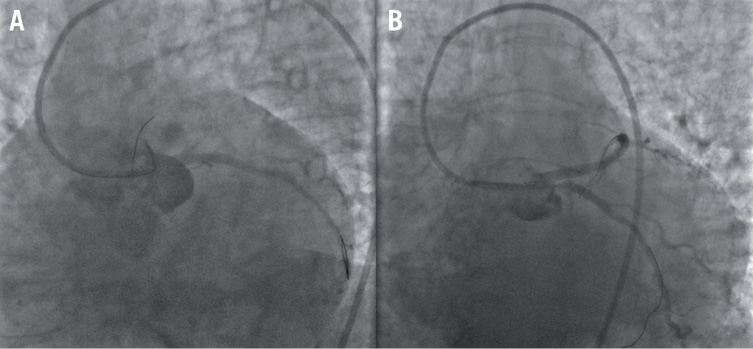
Figure 3. A) Complete occlusion of proximal left anterior descending artery, and B) proximal left anterior descending stenting (culotte stenting).

Figure 4. A and B) Kissing ballooning with (C) complete patency of left main, left anterior descending and left circumflex coronary artery.
The patient was discharged in a stable clinical condition, treated with ramipril, atorvastatin, clopidogrel, aspirin, beta blockers and furosemide. The peak troponin I level during hospital stay was 14.93 ng/ml. Two-month follow-up was uneventful.
To our knowledge, this is one of the first cases reporting “swinging” LM thrombosis complicating coronary angioplasty in an obese, diabetic, elderly woman treated with fondaparinux. Several results of apex format showed higher rates of fondaparinux-related catheter thrombosis1; this undesirable complication is even more threatening when involving the LM. In previous studies, fondaparinux was similar to enoxaparin for short-term efficacy, but reduced major bleeding by one-half and 30-day mortality by 17%1.
In a study enrolling more than 12,000 patients undergoing heart catheterisation, catheter thrombus was more common in patients receiving fondaparinux (0.9%) than enoxaparin alone (0.4%), but was largely prevented by using UFH at the time of PCI, without any increase in bleeding2. Fondaparinux however, even in combination with eptifibatide, was not sufficient to prevent cardiac catheter thrombus development in in vitro studies3. Nevertheless, the combination of fondaparinux with “half” therapeutic dosages of UFH were as efficient as other treatment strategies in preventing thrombus formation.
In this case report, fondaparinux is probably linked with LM thrombus formation. LM thrombosis progressively involved the LCx and, after LCx stenting, the LAD.
It remains unknown if the use of drug-eluting stents (DES) increases the risk of catheter-related coronary thrombosis in cases of fondaparinux administration. Coronary stenting with DES was shown to increase the risk of acute coronary thrombosis4. Results such as those that we report may therefore suggest the use of a possible routine administration of IIb-IIIa inhibitors or bivalirudin in patients treated with fondaparinux who need urgent PCI with DES. Catheter thrombosis has seldom been reported in any trial using bivalirudin5. Experimental systems show that polytherapeutic agents, including UFH and enoxaparin, seem to be more effective anticoagulants than certain single-target agents, such as fondaparinux6. More studies, however, are needed to assess whether catheter thrombosis is a class-, drug-, or dose-related effect, and how best to prevent it.
Conflict of interest statement
The authors have no conflict of interest to declare.
Online data supplement
Moving image 1. (A) Severe proximal left anterior descending coronary stenosis before, and (B) after angioplasty with drug-eluting stent.
Moving image 2. Massive thrombosis of the left main coronary artery, extending into the proximal left circumflex coronary artery.
Moving image 3. (A) Complete occlusion of the proximal left anterior descending artery, and (B) proximal left anterior descending stenting (culotte stenting).
Moving image 4. (A) Kissing ballooning with (B) complete patency of the left main, left anterior descending and left circumflex coronary arteries.