CASE SUMMARY
BACKGROUND: A 35-year-old man was referred to us in his third hour of severe retro-sternal pain, with ventricular fibrillation on paramedics’ arrival. No contraindications to pPCI were revealed on telephone referral. Direct questioning on cathlab admission, however, revealed the recurrent presence of small amounts of fresh blood in the stools, with the last episode two hours prior to admission.
INVESTIGATION: Physical examination, ECG, laboratory tests, coronary angiography, thrombophilia screening, VH-IVUS, cMRI.
DIAGNOSIS: Ectatic coronary arteries. Acute inferior STEMI as a result of thrombotic occlusion of RCA, with a very large thrombus burden. Recurrent, non-diagnosed, lower GI tract bleeding.
MANAGEMENT: ASA, clopidogrel, UFH, manual thrombectomy, balloon angioplasty, prolonged LMWH administration, control angiogram with intended culprit lesion stenting. Coronary CT-angiogram at 12 months.
KEYWORDS: acute coronary syndrome, non-controllable bleeding, ecstatic coronary arteries, large thrombus burden, manual thrombus aspiration, intracoronary drug administration, deterred culprit lesion stenting
PRESENTATION OF THE CASE
On a Sunday morning, a 35-year-old man (173 cm, 108 kg), heavy smoker (50 cigarettes per day), with known arterial hypertension, was referred to us by phone by the paramedic team that made a diagnosis of acute inferior myocardial infarction on the basis of symptoms and ECG. The patient had been woken up by retro-sternal pain whose intensity had been increasing for two hours when he made the emergency call. Ventricular fibrillation (VF) occurred on the paramedic’s arrival, which was successfully defibrillated. No contraindications to pPCI were revealed and the patient was accepted for interventional management. In the ambulance, aspirin (300 mg p.o.), clopidogrel (600 mg p.o.), UFH (5000 IU i.v.), morphine (5 mg i.v.) and amiodarone (300 mg i.v) were administered. The transfer time was 18 minutes.
On cathlab arrival, the patient appeared clearly unwell, sweating and distressed. ECG was consistent with a high-risk acute inferior STEMI (Figure 1A, note reciprocal ST depression in I, aVL, V1-V3). No relevant medical history was noted other than recurrent episodes of small amounts of fresh blood in stools that only became disclosed on direct questioning. These had occurred irregularly over a few years, with the last episode on the morning of the day of admission. The patient believed that he had anal varices but, against the advice of his GP, he had repeatedly refused any further diagnosis. Gross physical examination showed obesity. Per rectum examination revealed a trace of fresh blood.
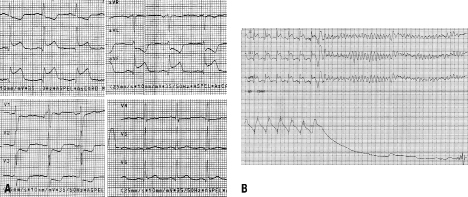
Figure 1. 12-lead ECG on admission showing a high-risk inferior STEMI (A), and 3-lead ECG immediately prior to coronary angiography showing VF (B).
Immediately prior to angiography there was another episode of VF (Figure 1B). This was resistant to defibrillation (a total of five shocks were required). An additional dose of amiodarone (300 mg i.v.) was administered and potassium infusion (40 mmol; baseline serum level of 3.9 ml/L) was started. Coronary angiogram (femoral access) showed ectatic arteries with no significant lumen reductions in the left coronary system (Figure 2A), and a total thrombotic occlusion of the proximal right coronary artery (Figure 2B).
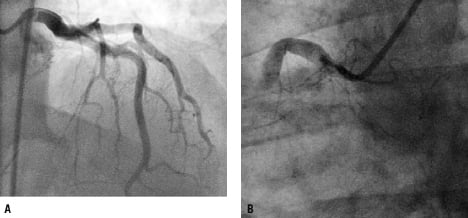
Figure 2. Coronary angiogram illustrating ectatic arteries without focal lumen reductions in the left coronary artery (A, 6 Fr JL catheter) and a total occlusion in the proximal RCA (B, 7 Fr JR catheter).
The following therapeutic strategies (alone or in combination) were considered:
– intracoronary lytic therapy with immediate or delayed percutaneous intervention
– intracoronary heparin and IIb/IIIa inhibitor infusion with immediate or delayed intervention
– manual thrombus aspiration
– “undersized” balloon angioplasty after pharmacologic and/or mechanical thrombus burden reduction
– thrombus burden reduction followed by “direct” stenting with/without an embolic protection device
THE INVITED EXPERT’S OPINION
The challenge in the treatment of the above patient who presented with STEMI is the presence of a large thrombus burden in the RCA of this young patient who presented earlier with lower gastrointestinal bleeding due to haemorrhoids. Management of coronary thrombus in this setting can be approached in two ways, either mechanical or pharmacological. The mechanical approach includes aspiration using an aspiration catheter, mechanical thrombectomy using the Possis device (Possis Medical, Minneapolis, MN, USA), or vaporising the thrombus using laser angioplasty. The pharmacological approach should use bivalirudin, which demonstrated superiority over heparin for patients presented with STEMI.1 The use of IIb/IIIa inhibitors could be synergistic agent to thrombin inhibitors for the reduction of the thrombus burden. However, in this specific case due to the history of bleeding, the use of aggressive pharmacological regimen can result in excessive bleeding. Therefore, in this specific case, the preference would be to focus on an aggressive mechanical approach, refraining from the use of IIb/IIIa inhibitors. One such approach, not proven in clinical trials, could be the local infusion of IIb/IIIa inhibitors via a perfusion catheter, minimising the risk of bleeding. Regardless, the patient should be loaded with clopidogrel 600 mg and aspirin 325 mg prior to the intervention.
Another consideration with a high thrombus burden is the possible risk for slow flow due to distal embolisation. This unwarranted complication can be minimised mechanically by placing filter or occluders distal protection device to prevent distal embolisation, and although this was not a proven strategy for all STEMI as shown in the EMERALD trial2, this approach should be considered in this case. In addition, the use of intracoronary nitropruside or nicardipine to minimise the slow flow phenomenon should be considered.
In the event that sufficient reperfusion is obtained, the lesion should be treated with direct stenting using bare metallic stent. The use of BMS is justified in this patient because he may have a limitation for prolonged dual antiplatelet therapy due to the lower GI tract bleeding, the fact that the vessel is large in diameter, and the probability of restenosis is low.
Conflict of interest statement
The author has no conflict of interest to declare.
How would I treat?
INVITED EXPERT’S OPINION
Prompt reperfusion is paramount for this young man with a high risk inferior ST elevation myocardial infarction accompanied by haemodynamic compromise and recurrent ventricular arrhythmias: a mechanical, angiographic guided strategy is clearly preferable in the presence of background lower gastrointestinal bleeding of an undefined source. There is certainly no role for systemic thrombolysis.
The rapid and effective out of hospital care provided by the Polish paramedic service is impressive – we pioneered pre-hospital administration of clopidogrel for the UK in Oxford and also use a high dose loading regime, consistent with data from ARMYDA-2 and ISAR CHOICE.1,2 Primary angioplasty is systematically performed via the radial approach to reduce overall risk of bleeding complications, and in this case I would have administered a low dose of unfractionated heparin following sheath insertion (3-5000 iU, maximum 70 iU/kg) as a prelude to coronary angiography.
The heavy thrombus burden within the large ectatic right coronary artery presents a significant challenge with high risk of an adverse outcome associated with no reflow. A stepwise minimalist approach dictated by events at each stage with the target of restoring adequate flow and ST normalisation is therefore the priority. Initial improvement in flow is likely after passage of a guidewire and low profile un-inflated balloon – if not, then low pressure inflation at the site of occlusion is usually helpful. Once the vascular anatomy is demonstrated, I would then routinely use a simple manual thrombus aspiration catheter, the acute and long term benefits of which have been demonstrated in the landmark TAPAS trial.3,4 Meta-analyses have demonstrated little benefit with more complex mechanical devices5 though recent anecdotal experience suggests that larger lumen aspiration catheters (e.g., Hunter) are more efficacious in cases where there is heavy thrombus burden.
Further management will be dictated by the angiographic appearances in the wake of thrombus extraction. Complete improvement seems unlikely and I would have a low threshold for administration of intracoronary abciximab (without an associated 12 hour systemic infusion). Acute effects on the microcirculation will be beneficial and the overall risk of important life threatening bleeding is low. Further mechanical intervention should be restricted within the original remit of flow restoration and preceded by administration of a high dose intracoronary vasodilator (adenosine, verapamil or sodium nitroprusside).6,7 If sufficient, a balloon-only strategy would be very reasonable, and stenting should be restricted to the minimum number and length possible (with maximum safe initial diameter and avoidance of high pressure post-dilatation). Although laser has a potential niche role in this setting, the technology and expertise are not widely available and the associated evidence controversial.8 Good luck!
Conflict of interest statement
The author has no conflict of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
A right Judkins 7 Fr guiding catheter was used and the culprit artery was wired with a BMW 0.014J guidewire. This revealed a very large thrombus in an ectatic vessel (reference diameter 6-7 mm) with a poor, sluggish flow through the lesion (TIMI 1, Figure 3A). Large thrombus burden in a large diameter vessel is a well-known risk factor of distal embolisation, no-flow and significant myocardial injury.1 A large intracoronary thrombus in the IRA can be managed in either a pharmacological, mechanical or combined way. Due to the particularly large size of the thrombus, intracoronary lytic therapy (alteplase or tenecteplase) would have been our first-line treatment since this can effectively reduce the thrombus burden prior to any further mechanical management.2-4 This strategy, however, was not pursued due to the unexplained and potentially uncontrollable bleeding in our patient. The presence of anal varices would not be an absolute contraindication to lytic therapy because the potential bleeding could be controlled with spongostan or, if necessary, by surgical ligation. However, a different cause of lower GI tract bleeding, making it uncontrollable (e.g., ulcerative colitis, polyps/cancer, or diverticula), could not be excluded on an ad hoc basis. For this reason, intracoronary abciximab was not administered either. Although platelet glycoprotein IIb/IIIa receptor antagonists are not lytic agents, evidence indicates that their administration can help shift the balance of thrombus formation/endogenous lysis towards the latter, reducing the thrombus burden and the risk of no-flow, and reducing the infarct size.5,6
We proceeded to mechanical intervention. The intracoronary route of heparin administration was chosen because this allowed us to achieve local drug concentrations several-fold higher than those achieved with intravenous delivery.7,8 Manual thrombus aspiration, our set routine in pPCI, was performed because this has been shown to effectively reduce thrombus burden, leading to a decrease in infarct size and mortality.9,10 A 7 Fr aspiration catheter was used because, for the Export catheter (Medtronic, Minneapolis, MN, USA) the aspiration capacity dramatically increases with a change of the catheter diameter from 6 Fr to 7 Fr (45 cc/min vs. 90 cc/min). Prior to manual aspiration (Figure 3B), an additional 5000 IU UFH was administered to the IRA. However, control ACT was only 168 sec, and another 14,000 IU UFH in fractionated doses (i.e., a total of 24,000 IU) was required to achieve the therapeutic ACT of 255 sec. Figure 3C shows thrombi aspirated with three passages of 7 Fr Export catheter in this procedure, and Figure 3D shows the angiographic result of manual thrombectomy. At that point, we considered two options. In the light of TIMI 3 IRA flow and symptom resolution, one strategy was to terminate the index PCI procedure and to maintain intravenous infusion of UFH (dose adjusted according to APTT) with close patient monitoring and a scheduled angiographic control at 24-48 hours or earlier in case of recurrent chest pain/ST elevation/haemodynamic instability. However, the residual thrombus burden was still substantial (Figure 3D), and the risk of “spontaneous” IRA reocclusion after vessel de-wiring was considered high. Therefore, we decided to proceed with mechanical intervention. Direct thrombus aspiration via the guiding catheter was previously reported11, but we decided against this for two reasons: (i) the presumed high risk of the ectatic IRA dissection or perforation with the 7 Fr guiding deep intubation, and (ii) the risk of extra-cardiac embolic complications (such as embolic stroke) on retraction of the guiding catheter which might have the large IRA thrombus “hanging” at its distal end. We accepted that distal IRA embolisation with possible no-reflow might occur as the cost for the index lesion further angioplasty. Theoretically, the deleterious effect of mobilising the large residual thrombus with stent implantation could be offset, at least in part, by using a protection device. The segment of RCA proximal to the thrombotic lesion (Figure 3D) was favourable as the landing zone for a proximal protection system with which we have experience in coronary and carotid interventions.12 Routine use of proximal or distal (filters or distal balloon occlusion) protection devices in STEMI did not translate into an angiographic, enzymatic (infarct size) or clinical benefit.13,14 However, there is circumstantial evidence that in lesions with a particularly large thrombus burden this strategy can lead to a reduced occurrence of the no-flow phenomenon and infarct size reduction.15 Direct stenting with an undersized coronary stent or a 0.014 guidewire compatible RX peripheral stent (e.g., a cobalt-chromium RX Herculink Elite® 6.5×18 mm [Abbott, Abbott Park, IL, USA]) was considered, but it was dismissed as being consistent with the idea that stent placement is inadvisable when the thrombus burden is heavy and the risk of stent deployment-associated distal embolisation and no-flow is very high through the “cheese-grater” effect.16
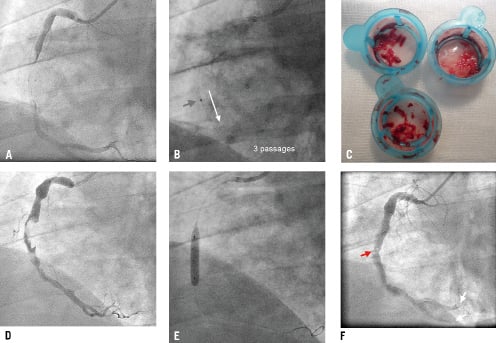
Figure 3. The percutaneous intervention. Lesion crossing with a BMW 0.014J guidewire revealed an extremely large thrombus in the culprit artery (A; Moving image 3A). Manual thrombus aspiration (7 Fr Export catheter) was performed (B, red arrow indicates the aspiration catheter marker), and multiple red thrombus fragments were removed (C). The angiographic result of thrombus aspiration is shown in D (Moving image 3D); there was TIMI 3 flow but the residual thrombus burden was very high. Undersized balloon (4.0×20 mm) low-pressure angioplasty was performed (E) to prevent infarct-related artery re-occlusion after the guidewire removal. F shows the final result of the index procedure (floating residual thrombi at the index lesion site are marked by the red arrow, whereas white arrows indicate distal embolisation; Moving image 3F).
A decision was made to perform low-pressure undersized balloon angioplasty to increase the lumen size at the index lesion. Consistent with the guideline-recognised17 role of adenosine in pharmacological no-reflow prevention, we administered 3 intracoronary boluses of 60 μg adenosine prior to a low-pressure inflation with an undersized balloon (4.0×20 mm, 6 atm, Figure 3E). Balloon angioplasty led to an effective index lesion dilatation without angiographic evidence of dissection (Figure 3F). This, however, occurred at the cost of distal embolisation (Figure 3F, white arrows) and deterioration of the IRA flow (TIMI-2; increase in cTFC from 38 after thrombus aspiration to 100 after balloon angioplasty; cf., cTFC of 58 in the ectatic, fully patent, LAD). Further application of adenosine (3×60 μg) and sodium nitroprusside (3×100 μg) did not improve the epicardial (TIMI 2) or myocardial (cTFC=100) flow, but the IRA was patent and the patient was stable haemodynamically and electrically, with only residual retro-sternal pain. UFH was maintained for 24 hours (dose-adjusted to APTT) and, after sheath removal, low-dose enoxaparin (100 mg o.d. s.c.) was started18 on top of ASA, clopidogrel, ACEI, ß-blocker, and a high-dose statin.17 Angiographic control was scheduled for day 6-7 (earlier in case of ischaemic complications), with the intention of culprit lesion stenting if required. However, the patient self-discharged at day five. Peak troponin I was 21.34 ng/mL (N <0.01 ng/mL) and peak CK-MB was 182 U/L (N<24U/L). Screening for thrombophila including Factor V Leiden,prothrombin 20210A mutation, anti-phospholipid antibodies, protein C deficiency, protein S deficiency or antithrombin deficiency was negative. Plasma total homocystine level was normal. Nevertheless, the patient turned out to be a homozygote for F XIII Leu34Leu, a variant that is seen in ≈4% of the white population and is associated with the formation of dense and lysis-resistant fibrin clots.19 This finding might partially explain the particularly large thrombus formed in the infarct-related artery in this patient.
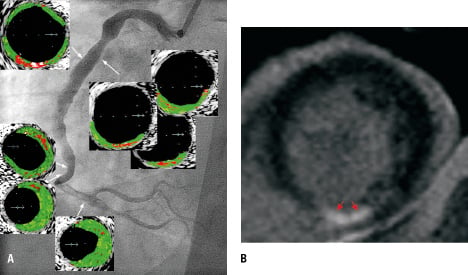
Figure 4. Control angiogram of the culprit artery showing an optimal angiographic result (A, Moving image 4A1 and 4A2). VH-IVUS indicated atherosclerotic lesions, with fragments of necrotic core appearing to be in contact with the lumen (A), MRI study that revealed only small zones (B, red arrows) of late gadolinium enhancement, with the total infarct mass of 5.2 g, i.e., 2.85% of the total LV mass of 183 g. Moving images 4C1 and 4C2 shows LV contractility by cMRI at four weeks.
Follow-up
Mechanical stabilisation of the STEMI culprit lesion with a stent is the recommended strategy in pPCI.17 Four weeks after the index procedure, we convinced the patient to undergo a control angiogram with the intention to stent the index lesion in case of a suboptimal pPCI result in this early follow-up. However, the control angiogram showed an optimal effect of the index balloon angioplasty (Figure 4A). On this occasion, VH-IVUS was performed; this showed atherosclerotic plaques within the ectatic segments. Proximal to the index lesion there were necrotic core areas that appeared (by the VH-IVUS; resolution of 100-150 μm) to be in contact with the vessel lumen. Coronary ectasiae, irregular saccular vessel dilatations, were believed to be caused by genetic connective tissue abnormalities and to provide the basis for acute coronary syndromes by abnormal flow dynamics (sluggish, disturbed, non-linear flow) rather than by atherosclerotic plaque rupture.20 For this reason, long-term anticoagulation was thought to provide optimal medical treatment.20 However, consistent with the present report (Figure 4A), recent data indicate that ectasiae can contain highly-inflamed atherosclerotic plaques with the proclivity to rupture.21
Cardiac MRI showed mild inferior wall hypokinesia with mildly reduced systolic muscle thickening and a mild reduction in overall LV contractility (LVEF 54%). Infarct size by gadolinium late-enhanced mass (Figure 4B) was 5.2 g (2.85% of the total LV mass of 183 g), consistent with only a small myocardial injury. The patient was asymptomatic and denied any bleeding from the lower GI tract within the previous month. Antiplatelet therapy included ASA 100 mg o.d. (indefinitely) and clopidogrel 75 mg o.d. for 12 months.17
Three months later (i.e., four months after STEMI) the patient presented in a local general hospital due to progressive weakness. Basic laboratory tests showed anaemia (Hb 8.6 g/dL, RBC 3.5×106 [“power 6”] rather than RBC 3.5×106, Hct 29%). Clopidogrel was stopped while ASA was maintained. The patient continued to refuse any lower GI tract diagnosing and, again, he self-discharged. However, at 12 months he presented in our outpatient clinic with non-specific chest pain in absence of anaemia (Hb 14.3 g/dL). The exercise ECG test was negative by the ECG criteria but the patient reported chest discomfort. To exclude any significant progression of CAD, CT-angiogram was performed (Figures 5A and 5B). This showed ectatic coronary arteries without any significant lumen reductions, consistent with the control angiogram at four weeks after STEMI.
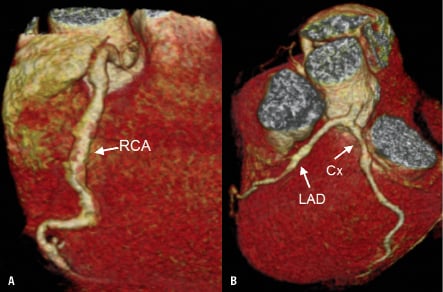
Figure 5. CT angiogram at 12 months, showing ectatic coronary arteries without significant lumen reductions.
Conclusion
The patient with a high-risk STEMI coexisting with potentially uncontrollable bleeding poses specific diagnostic and therapeutic challenges in the context of a situation that requires rapid decision-making. The present case shows that individualised treatment strategies need to be considered in clinical scenarios not fully covered by the guidelines.
Manual thrombus aspiration is the first-line management of thrombus-containing lesions. In selected patients, the stent placement can, at least temporarily, be deterred if the residual thrombus burden remains high. In such cases, according to circumstantial evidence, a prolonged antithrombotic treatment with LMWH on top of antiplatelet regimens might be beneficial by facilitating a further thrombus burden reduction.
Finally, in cases of a substantial residual thrombus burden after thrombus aspiration, there may be an important role for novel low-profile ‘cotton wool’-like atraumatic high-efficiency filter systems (such as FiberNet®, Medtronic-Invatec, Minneapolis, MN, USA)22 and for implantation of a mesh-covered stent that is designed to ‘trap’ the remaining thrombus/plaque between the stent and the vessel wall (e.g., MGuard®, InspireMD, Tel Aviv, Israel).22 These systems were not yet commercially available when we performed the procedure reported herein.
Conflict of interest statement
The authors have no conflict of interest to declare.
Online data supplement
Moving image 3A. Occlusion crossing with a BMW 0.014J guidewire revealed an extremely large thrombus burden in the ectatic RCA.
Moving image 3D. Manual thrombus aspiration with a 7F Export catheter removed large amounts of fresh thrombus and it resulted in TIMI 3 flow in the IRA, but the residual thrombus burden was still extremely high suggesting a threatened IRA re-occlusion if the guidewire were to be removed at this point.
Moving image 3F shows the final angiographic result of pPCI. Note the floating residual thrombi at the index lesion site, distal embolisation, and a reduced TIMI flow (TIMI 2) that did not improve after adenosine and sodium nitroprusside intracoronary administration.
Moving images 4C1 and 4C2 show LV contractility by cMRI at four weeks.

