CASE SUMMARY
BACKGROUND: A 53-year-old man symptomatic for unstable angina, underwent PCI for a severe stenosis of the first obtuse marginal and a CTO of the left circumflex arteries.
INVESTIGATIONS: Physical examination, myocardial necrosis markers, ECG, transthoracic echocardiography, exercise ECG test, bilateral coronary angiography, cardiac magnetic resonance.
DIAGNOSIS: During PCI, antegrade contrast injection displaced a large clot from the guiding catheter into the left coronary artery causing massive thrombosis. The patient became haemodynamically unstable. The pressure wave from the guiding catheter was damped.
MANAGEMENT: Intravenous UFH and abciximab followed by aspiration from the guiding catheter, and then through an aspiration catheter, until clear blood came out and pressure wave was normalised. Subsequent left coronary angiography showed no residual thrombi with TIMI-3 flow. Afterwards, a CMR scan showed no myocardial damage.
KEYWORDS: antithrombotic therapy, chronic total occlusion complication, coronary catheter thrombosis, thrombus aspiration, cardiac magnetic resonance
PRESENTATION OF THE CASE
A 53-year-old male patient, obese (weight 82 Kg, height 159 cm), hypertensive and with a family history positive for ischaemic heart disease, was admitted to our emergency room for unstable angina. ECG showed negative T waves in the inferior-lateral leads. Transthoracic echocardiography showed a normal left ventricular (LV) size and function with a LV ejection fraction 58%. Routine myocardial necrosis markers (creatine kinase (CK), CK-MB and high sensitivity troponin T) were negative.
Recent medical history was characterised by increasing exertional chest pain four months before hospital admission. In order to establish the diagnosis, an exercise ECG test was performed showing 3 mm ST depression in the inferior-lateral leads with typical chest pain. We therefore decided to perform coronary angiography which showed severe stenosis of the proximal segment of the first obtuse marginal branch (OM1) and chronic total occlusion (CTO) of the left circumflex artery (LCx) after OM1 take-off (Figure 1). CTO features were:
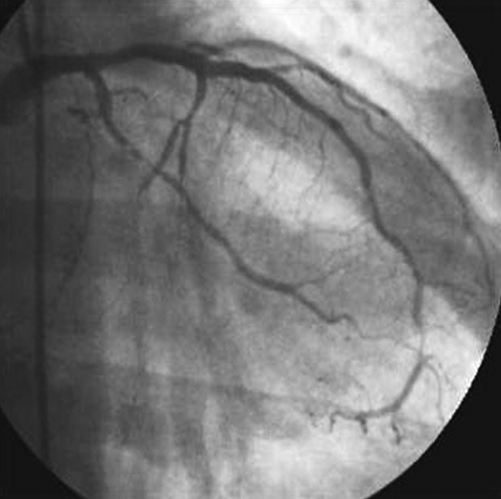
Figure 1. Severe stenosis of proximal segment of OM1 and chronic CTO of LCx after OM1 take-off.
– OM2 retrograde filling from posterior-lateral branch of right coronary artery (RCA), with faint antegrade collateral circulation (Figure 2).
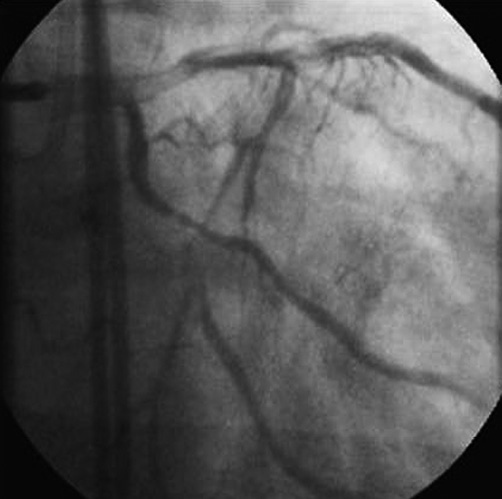
Figure 2. Angiography showed OM2 retrograde filling from posterior-lateral branch of RCA, with faint antegrade collateral circulation. Occlusion length was around 12 mm with a blunt stump and side branch take-off at the level of proximal cap (OM1).
– Blunt stump
– Side branch (OM1) at the level of proximal cap
– Occlusion length around 12 mm.
Considering the clinical and diagnostic findings and the large jeopardised myocardial area, we decided to perform PCI of OM1 stenosis and LCx CTO.
After a bilateral femoral artery approach, intubation of the left main coronary artery (LMCA) and the right coronary artery was obtained with XB 3.5 7 Fr and JR4 7 Fr guiding catheters, respectively. An intracoronary bolus of 7,000 IU of UFH was then administrated and a HI-TORQUE PILOT 50 guidewire (Abbott Vascular, Abbott Park, IL, USA) was advanced into the distal OM1 without difficulty. After that, we commenced the antegrade recanalisation of the CTO with a Miracle 4.5 guidewire followed by a Conquest Pro 12 guidewire (ASAHI Intecc, Aichi, Japan) through a FineCross™ microcatheter (Terumo Corp., Tokyo, Japan). After crossing the lesion, contrast injection through the microcatheter showed an intimal dissection in OM2 (Figure 3). We then switched to the STAR technique: the Conquest Pro 12 was exchanged for an umbrella-shaped PILOT 50 guidewire that was advanced into the true lumen with the support of the microcatheter. Contrast injection via the microcatheter confirmed the wire into the true lumen of OM2 (Figure 4). The procedure lasted about 45 minutes.
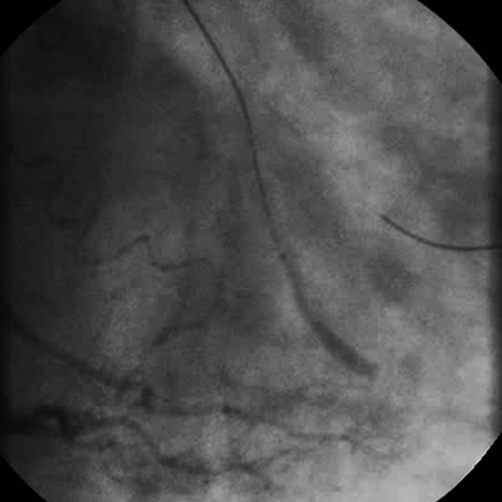
Figure 3. Contrast injection through the microcatheter showed OM2 intimal dissection.
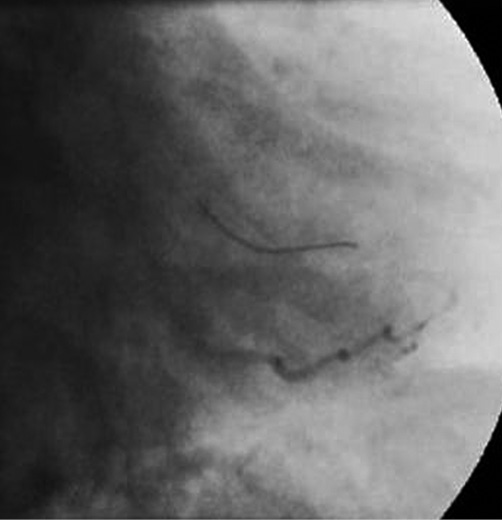
Figure 4. After a switch to the STAR technique with an umbrella-shaped PILOT 50 guidewire advanced with the support of the microcatheter, contrast injection via the microcatheter confirmed the access to the true lumen of OM2.
After withdrawal of the microcatheter, in order to plan the subsequent procedural step, a left coronary angiogram was taken which showed a massive thrombosis of LMCA involving proximal LAD, diagonal branch and LCx (Figure 5). The patient started to complain of chest pain, became hypotensive (arterial blood pressure 84/46 mmHg), and irritable. The pressure wave from the guiding catheter was damped and monitor ECG tracing showed marked ST-segment depression.
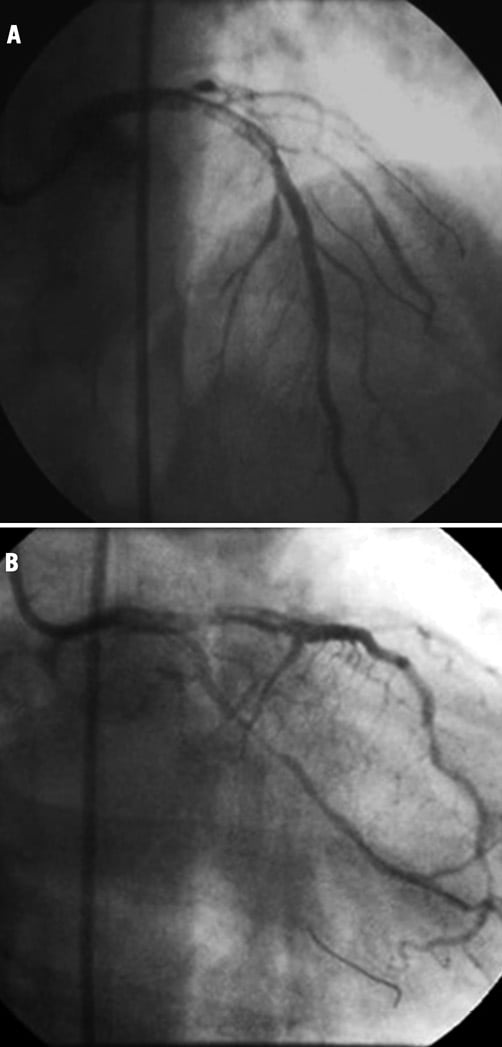
Figure 5. (A and B) Massive thrombosis of LMCA involving proximal LAD, diagonal branch and LCx.
How would I treat?
THE INVITED EXPERT’S OPINIONJavier Escaned*, MD, PhD
Coronary thrombosis is one of the most feared complications of chronic total occlusion (CTO) recanalisation. Its occurrence is favoured by the duration of this type of procedure and by the heavy instrumentation of the vessels, in which intracoronary devices, such as microcatheters, anchoring balloons, Tornus, Corsair or IVUS catheters, have to remain for long periods of time inside the coronary arteries. In addition to this, due to the concentration demanded by the complexity of the case, the operator may forget to flush the guiding and diagnostic catheters regularly. Some operators are reluctant to initiate double antiplatelet therapy (DAPT) before crossing the CTO because they fear that coronary perforation might be more difficult to control when thienopyridine treatment has been initiated, and this may also increase the chances of intraprocedural thrombosis. Finally, the consequences of coronary thrombosis may be dramatic if the involved vessel is a collateral donor to that with CTO or, as in this case, if the main stem becomes compromised1,2.
So, in terms of prevention of this complication in CTO cases, my personal attitude would be 1) to initiate DAPT before the procedure; 2) to ensure that the activated clotting time remains above 300 seconds throughout the procedure by checking it every 20 min (and to correct it with additional boluses of heparin if required); and 3) to flush guiding and diagnostic catheters regularly. As part of CTO teamwork, some of these responsibilities (such as ACT monitoring) should be assigned to the nursing personnel, less engaged in the attention-demanding tasks carried out by the operators.
If coronary thrombosis develops and, as in this case, it constitutes a vital threat to the patient, my personal attitude would be to focus on its solution by percutaneous and pharmacological means. The thrombus observed in the LM and LAD can have an origin such as 1) thrombosis in situ (LM/LAD/OM) as a consequence of suboptimal antithrombotic treatment and disturbed haemorrheology by intracoronary instruments; 2) an embolus originated in the guiding catheter; and 3) thrombotic material formed in the LCx subintimal space and accidentally retrieved during the removal of the microcathether. From my point of view, the angiographic pattern in this case seems suggestive of thrombosis in situ. Irrespective of its origin, the first urgent actions I would take would be 1) to administer a bolus of intracoronary abciximab, followed by intravenous infusion; 2) to secure access to the distal LAD with a new guidewire; and 3) to perform manual thrombus aspiration over the LM/LAD and OM wires. In the meantime, other actions should be initiated: ACT should be checked to assess the status of anticoagulation, and intra-aortic balloon pumping should be prepared to be inserted through the contralateral femoral artery sheath.
Administration of abciximab is not contraindicated since at the time of the thrombotic event there was no evidence of coronary perforation. Besides, as mentioned above, abciximab is a key therapeutic element in a life-threatening complication and its use takes priority in this situation. Balloon dilation and stenting should be sequentially considered in case thrombus aspiration failed to restore coronary flow in the LM and LAD. Correction of any precipitating factor identified (e.g., suboptimal anticoagulation diagnosed by ACT) should be mandatory. Again, teamwork is a key element in the success of these treatments.
In general, should the situation be controlled with these measures, with adequate restoration of blood flow in the LM, LAD and first obtuse marginal, I would stop the procedure in the LCx, and consider a new attempt in a separate procedure if deemed necessary. Echocardiographic assessment immediately after the procedure and over the following hours should be performed to rule out the development of slowly progressing pericardial effusion as an unlikely but potential complication of abciximab administration in this patient.
Conflict of interest statement
The author has no conflict of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
Geraci and colleagues reported a case of massive thrombus embolisation during chronic total occlusion revascularisation in a patient with unstable angina. Although the overall incidence of this dreadful complication is unknown, mainly because of under-reporting, it occurs rarely during elective PCI procedures, and the treatment strategy is challenging and not always effective. Long procedural time, complex procedures and inappropriate use of antiplatelet and anticoagulant drugs are important predictors of periprocedural intracoronary thrombosis1,2. The best treatment strategy of thrombus embolisation is to prevent it whenever possible. Pretreatment with dual antiplatelet therapy (i.e., aspirin and 600 mg clopidogrel) and intravenous administration of 100 IU/kg of unfractionated heparin (UFH) should be the standard of care3. Moreover, particularly in complex and time-consuming procedures, frequent control of the activated clotting time (ACT) should be mandatory.
In this specific case, massive thrombosis of the LMCA involving the proximal LAD, diagonal branch and LCx developed during a CTO procedure. This complication probably occurred as a consequence of thrombus embolisation from the guiding catheter. A well-defined treatment algorithm to deal with coronary thrombosis has not been defined. Therapeutic options include pharmacological therapy, such as thrombolysis and anticoagulation, and mechanical thrombus aspiration. Often, they consist of a combination of different therapeutic acts. An immediate invasive approach is necessary since the patient had already experienced symptoms and hypotension, associated with ischaemic ECG changes. In this situation, since the LMCA is also involved, particular attention should be paid in order to avoid cerebral embolism1. As a first step, vigorous blood aspiration through the guiding catheter should be performed since pressure damping might indicate the presence of thrombus at the catheter’s tip. As a second step, we would ensure adequate medical management of both symptoms (i.e., morphine) and hypotension (saline infusion to start with) and, if necessary, correct the ACT (an ACT of at least 250 seconds should be targeted) using additional unfractionated heparin. The thrombus burden in this patient is large and we would therefore proceed with a thrombus aspiration system. Manual aspiration devices are preferable as they are easy to use, they remove thrombus material effectively and they improve clinical outcome, as documented in patients presenting with ST-segment elevation myocardial infarction4. Mechanical thrombectomy devices, such as the AngioJet® system (Medrad Interventional/Possis, Minneapolis, MN, USA) are useful especially in situations with large thrombus burden. However, they are more complex to operate and do not unequivocally improve clinical outcome5,6. Further management of this case would depend on the residual thrombus burden. If the thrombus had been removed sufficiently and adequate flow in the left coronary artery had been ensured, we would pursue the treatment of the CTO as scheduled as the two wires are in the true lumen. In case of persistent thrombosis and impaired flow, we would administer intracoronary abciximab followed by intravenous infusion for at least 12 hours as it reduces thrombus size and improves myocardial perfusion7. In the latter situation, we would bring the patient back for an angiographic control within 72 hours, to address persistent thrombus left untreated. We would leave the CTO untreated for at least four weeks to allow optimal vessel healing8.
Conflict of interest statement
The authors have no conflict of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
At this point activated clotting time (ACT) was 260 seconds. An extra bolus of 5,000 IU UFH and a bolus of the glycoprotein IIb/IIIa inhibitor abciximab were administered intravenously. Blood aspiration was immediately performed from the XB guiding catheter, then through an aspiration catheter (Pronto V3; Vascular Solutions Inc., Minneapolis, MN, USA). Aspiration continued till free clear blood came out of the aspiration catheter (about 100 cc) and the pressure wave was normalised. The guiding catheter was then removed under continuous aspiration and, after retrieval, was flushed with expulsion of massive catheter-shaped clots (Figure 6). A new guiding catheter was rapidly exchanged on coronary guidewires and used for angiographic assessment of the left coronary system.
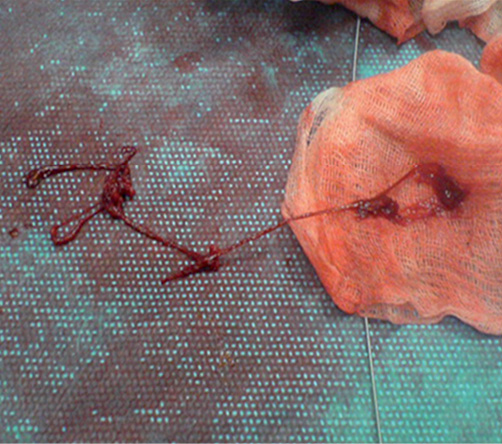
Figure 6. Massive catheter-shaped clots expelled from the guiding catheter.
Final angiography showed no residual thrombi with normal flow throughout the left coronary system (TIMI-3 flow) (Figure 7). The patient became free of chest pain, with good improvement of blood pressure (110/70 mmHg) and was then admitted to the intensive coronary care unit, with intravenous infusion of abciximab for 12 hours. CTO recanalisation of OM2 was postponed. During the hospitalisation, congenital thrombophilia, other common forms of acquired thrombophilia were excluded.
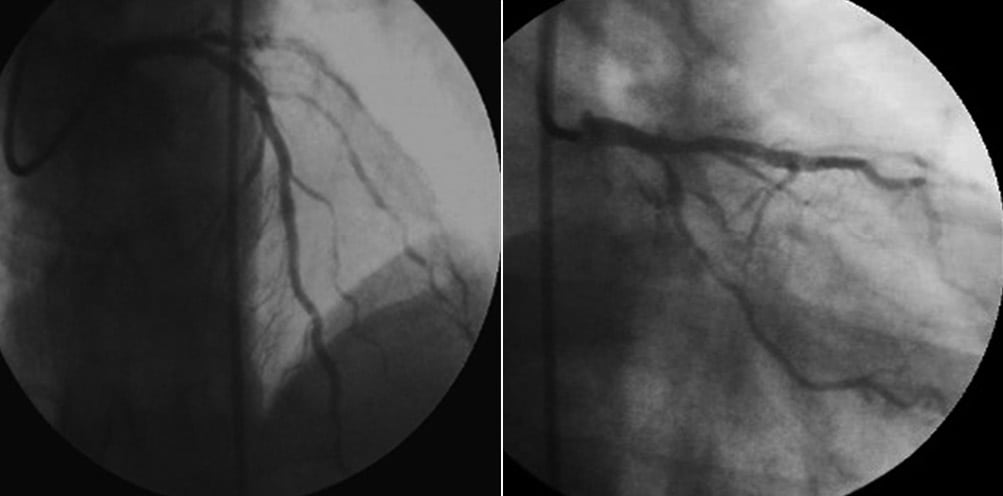
Figure 7. Final angiography showed no residual thrombi with normal flow in the left coronary artery.
The patient was discharged three days later asymptomatic, with normal ECG and cardiac biomarkers.
Cardiovascular magnetic resonance was performed before discharge, showing normal LV function and confirming the absence of periprocedural myocardial necrosis on late gadolinium enhancement imaging (Figure 8).
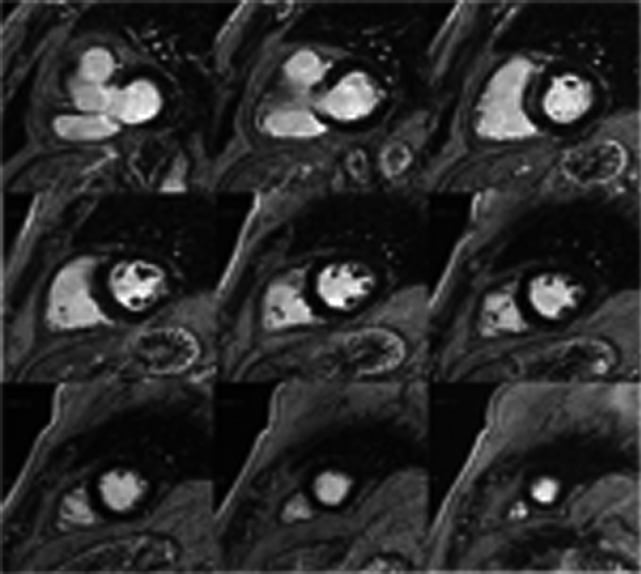
Figure 8. Cardiovascular magnetic resonance before discharge showed normal LV function and absence of myocardial necrosis on late gadolinium enhancement imaging.
Chronic total occlusions (CTOs) are a relatively common finding identified in up to 15% of all patients referred for coronary angiography1; nevertheless, their treatment is attempted in only 8% to 15% of patients undergoing percutaneous coronary intervention (PCI)2,3.
Chronic total occlusions represent a hard challenge for interventional cardiologists and they have been referred to as the final frontier in interventional cardiology4.
The difference between the frequency of CTOs and their percutaneous treatment is related not only to the procedural complexities of this lesion subset, but also to the uncertainties with regard to patient selection: who could benefit from percutaneous treatment? Indeed, CTO does not represent a widely accepted indication for PCI. Despite the fact that there are consistent data on the benefits of successful CTO PCI with regard to symptom relief, the lesser need for surgical coronary revascularisation and improvement in both ventricular function and survival, these complex procedures are associated with lower rates of success, greater radiation exposure, longer procedure times and relatively higher complication rates compared to PCI in non-CTO lesions5-7.
Knowledge of the type, rate and characteristics of complications that may occur during CTO PCI is very important for evaluating the risk/benefit ratio of treating a CTO. We can identify two different groups of CTO-related complications: cardiac and extracardiac complications, this differentiation to be considered mostly didactic8.
In the first group, acute myocardial infarction, coronary dissection and perforation, cardiac tamponade, vessel and stent thrombosis are included; in the second one, aortic dissection, contrast induced nephropathy (CIN), vascular access site complications, radiation injury and stroke are listed. In the recent ERCTO registry, 1,914 patients with 1,983 CTO lesions were enrolled. The rates of complication were: coronary perforation 2.6%, with a cardiac tamponade related rate of 0.5%, vascular access site complications 0.7%, Q-wave myocardial infarction 0.1%, non-Q-wave myocardial infarction 1.2%, CIN 0.9% and, finally, the death rate was 0.3%9.
Coronary artery embolism is a rare complication of percutaneous coronary intervention10,11. Nevertheless, it should be considered in high-risk patients such as those undergoing long and complex percutaneous coronary intervention, e.g., CTO revascularisations. Although there is no consensus on optimal therapy for coronary embolism, reported therapeutic options include thrombolytic therapy, glycoprotein IIb/IIIa inhibitors, percutaneous coronary intervention (PCI) with or without stenting, catheter aspiration and embolectomy12,13.
During diagnostic coronary angiography or PCI, the risk of complications related to LMCA is low but, when a complication occurs, it tends to be life-threatening and to contribute in large part to the total catheter-related mortality14. Among the LMCA-related complications, acute occlusion is a very dangerous condition, especially in the setting of cardiogenic shock.
Manual thrombectomy by clot extraction with an aspiration catheter has been shown to reduce mortality in acute myocardial infarction (AMI)15. This case supports the evidence suggesting that the greatest benefit of thrombus aspiration occurs in patients with high thrombus burden16. Furthermore, an observational study showed that there is higher benefit of thrombus aspiration in patients with cardiogenic shock17.
In the present case, to our knowledge the first reported in medical literature for its features and context, we show how to manage an acute and potentially fatal complication, such as massive embolic occlusion of the left coronary artery, with a standard approach consisting of thrombus aspiration and intravenous administration of abciximab. It should be emphasised that in complex and long procedures, such as CTO revascularisations, involving multiple wires, catheters and other devices, it is mandatory to maintain an activated clotting time longer than 300 seconds, to check it every 30 minutes and, as often as possible, to keep vascular access and catheters clean in order to prevent thrombotic and thromboembolic events potentially fatal to the patient.
Conflict of interest statement
The authors have no conflicts of interests to declare.
Online data supplement
Moving image 1. Massive thrombosis of LMCA involving proximal LAD, diagonal branch and LCx.
Moving image 2. Final angiography showed no residual thrombi with normal flow in the left coronary artery.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Massive thrombosis of LMCA involving proximal LAD, diagonal branch and LCx.
Moving image 2. Final angiography showed no residual thrombi with normal flow in the left coronary artery.

