In this chapter of Tools and Techniques - Clinical, catheter compatibility in CTO recanalisation is discussed using a stepwise approach. The following is a summarised overview of this technique. The complete, unabridged version with images is available online at www.eurointervention.org
Background
Modern interventional cardiology is moving away from the use of large catheters, driven by the miniaturisation of intracoronary balloons and stents. The combination of dedicated microcatheters, intravascular ultrasound, re-entry devices and bilateral injection has become a standard in modern revascularisation of CTO.
In this report we analyse the compatibility of these materials inside different lumen guiding catheters.
Indication
Selection of the guiding catheter represents the first step for a successful CTO recanalisation. In fact, the correct guiding catheter should provide the necessary back-up force, support and stability throughout the whole procedure.
Two important considerations should be kept in mind for the optimal selection of a guiding catheter. The catheter shape is essential for providing optimal engagement of the artery and a coaxial orientation. The extra back-up shape is the most used for the left coronary engagement, especially if a radial approach is planned. Amplatz left curves are valid alternatives, mainly for left circumflex lesions. For the right coronary, Judkins right or Amplatz right represent the most employed catheters, while the Amplatz left curves are useful for shepherd’s crook origin engagement.
Selection of the right catheter size influences the type and combinations of devices allowed for the procedure. Moreover, bigger catheters provide higher passive back-up.
Difficulties
Despite optimal catheter selection and coronary engagement, sometimes an extra back-up support is needed. This could be reached with a deeper intubation, if a small catheter is selected, or anchoring the catheter to a small side branch. Alternatively, a mother and child device or a catheter extension can be employed.
In small coronaries or in very proximal and ostial lesions, catheter engagement may cause pressure damping or vessel dissection if a strong dye injection is performed. To avoid these events, guiding catheters with side holes are often indispensable, especially if a 7 Fr or 8 Fr catheter is selected.
Another important step during CTO recanalisation is represented by microcatheter or over-the-wire balloon (OTW) exchange, leaving the wire in place. If a trapping technique is planned, the right catheter size selection is mandatory.
Methods
We tested in vitro inside 5, 6, 7, 8 Fr guiding catheters (Launcher; Medtronic, Inc., Minneapolis, MN, USA) and inside 6 and 7 Fr GuideLiner® (Vascular Solutions, Minneapolis, MN, USA), frequently employed devices for CTO recanalisation such as new-generation OTW and monorail balloons, microcatheters, dedicated devices and IVUS probes. In the 6 Fr guiding catheters, all the dedicated CTO devices can be inserted alone. Only two end-hole support microcatheters or one microcatheter and a monorail balloon fit together (Figure 1). In the 7 Fr guiding catheters, many more devices can be used in combination including two Corsair microcatheters (Asahi Intecc, Aichi, Japan) and three smaller devices such as two Finecross® MG (Terumo Europe, Leuven, Belgium) and/or Valet® (Volcano, San Diego, CA, USA) and a monorail anchoring balloon. Inside an 8 Fr guiding catheter (Figure 2), all the devices can be inserted with the IVUS probe (Eagle Eye; Volcano, San Diego, CA, USA), including the Venture® (St. Jude Medical, Inc., St. Paul, MN, USA), as well as a wider array of two and three devices in combination which are also allowed.
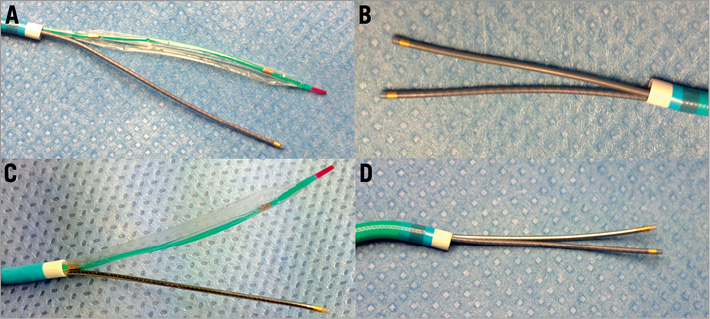
Figure 1. Compatibility of devices inside a 6 Fr guiding catheter. A) Combination of Finecross and monorail balloon; B) combination of two Finecross microcatheters; C) combination of Valet and monorail balloon; D) combination of Valet and Finecross.
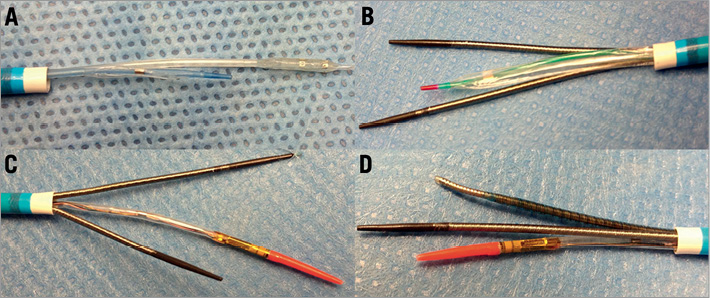
Figure 2. Compatibility of devices inside an 8 Fr guiding catheter. A) Combination of Stingray® (BridgePoint/Boston Scientific, Natick, MA, USA) and 1.25 mm OTW balloon; B) combination of two Corsairs and a monorail balloon; C) combination of two Corsairs and IVUS probe; D) combination of Venture and Corsair plus IVUS probe.
Acknowledgement
Abbott Vascular, Asahi, Boston Scientific, Medtronic, Biosensors have contributed to the sponsorship of the 4th EuroCTO annual meeting in London, for which some of the material presented in this article has been prepared.
Conflict of interest statement
The authors have no conflicts of interest to declare.

