Abstract
Aims: To evaluate the safety and efficacy of the CiTop™ Expander™ guidewire in attempting to cross through chronic total occlusion in CAD patients with various coronary dimensions and morphology. Although chronic total occlusions are encountered frequently in patients with coronary artery disease, an effective strategy to deal with them has yet to be devised. Various new guidewires have been designed in an attempt to negotiate chronic occlusions successfully. The aim of the CiTop™ Expander™guidewire is to improve the success rate of chronic total occlusion (CTO) recanalisation.
Methods and results: Ten consecutive male or female patients between 21 and 80 years of age, with no significant comorbidities and with angiographic documented chronic total occlusion (> 1 month) showing distal TIMI flow 0, or a prior failed guidewire attempted CTO were included in the study. The endpoints analysed were technical success (crossing of CTO by placement of CiTop™ Expander™ distal to occlusion with no device related major complications), angiographic success (<20% residual stenosis and TIMI flow grade 3), and clinical success. The basic features of the novel guidewire and its assessment of compatibility with other cathlab equipments were also recorded. The mean (± SD) age of the all male patient group was 53.6±9 years. The mean (±SD) lesion diameter and length was 3.1±0.4 mm and 20.4±7.9 mm, respectively, while the mean (±SD) age of occlusion was 25.5±26.8 months. Technical and angiographic successes were obtained in seven patients (70%). No events were recorded within seven days and 30-days follow-up after discharge.
Conclusions: The CiTop™ Expander™ guidewire was found to be efficacious and safe for use in recanalisation of chronically occluded coronary arteries in this initial experience.
Introduction
PCI (percutaneous coronary intervention) of chronic total occlusions (CTO) represent 10-20% of all angioplasty procedures and poses a management dilemma for the interventional cardiologists1. Although collaterals maintain myocardial viability under resting conditions, they often fail to provide sufficient blood flow during periods of increased oxygen demand, resulting in lifestyle limiting angina. Successful revascularisation may improve anginal status, increase exercise capacity, and reduce the need for late bypass surgery. However PCI of a CTO is associated with lower success rates, higher equipment costs, increased radiation exposure and more restenosis as compared to PCI of partial occlusion1.
Until recently, the clinical benefits of CTO recanalisation had not been clearly demonstrated, which, in concert with the technical complexity of the procedure, resulted in many patients with CTOs being either not treated or referred to bypass graft surgery. There is now an increasing body of published evidence demonstrating that successful percutaneous recanalisation of occluded vessels subtending viable myocardium not only reduces angina and improves quality of life, but also may improve left ventricular function and is strongly associated with enhanced survival2-4. Moreover, long term patency and freedom from restenosis after successful recanalisation of CTOs may be greatly enhanced by implantation of drug eluting stents5. The prospective, observational study (TOAST-GISE) reported that successful PCI was achieved in a high percentage of CTOs with a low incidence of complications. At one-year follow-up, patients with successful PCI of a CTO had a significantly better clinical outcome than patients where PCI was unsuccessful2. More recent data have also suggested a survival benefit from successful CTO revascularisation. Hoye et al6 showed that 5-year survival was significantly higher in patients with a successful CTO revascularisation (93.5% versus 88%) and Aziz et al7 showed that CTO revascularisation failure was an independent predictor of death. Furthermore, in patients with multivessel disease including a CTO, when complete percutaneous revascularisation is attempted, cardiac survival rate was higher in the CTO successful group as compared to CTO failure (91.6% versus 86.6%)8.
The CiTop™ Expander™ guidewire is a steerable 0.0014” guidewire with a deflectable tip. The CiTop™ Expander™ is a dedicated CTO guidewire designed for peripheral and coronary CTOs. We hereby report the first-in-man (FIM) experience with the CiTop™ Expander™ guidewire in 10 patients with coronary CTOs.
Methods
Patient population
Ten patients with angiographically documented chronic total occlusion (>1 month old) in coronary arteries, showing distal TIMI flow 0, and aged between 21 and 80 years with no significant comorbidities were included in the study. The patient unable to give informed consent, current participation in another study with any investigational drug or device, factors making follow-up and/or repeat angiography difficult or unlikely, contraindication to emergency artery by pass surgery, lack of surgical backup, contraindication to treatment with aspirin, or clopidogrel and/or heparin, lesion >40 mm in length (both calcified lesion and adjacent thrombus), treated vessel reference diameter less than 2.5 mm, visualisation of the distal lumen less than the Rentrop Classification Grade 2 collateralisation, non-visible entry point-of-target lesion, totally occluded bypass graft as target vessel, acute MI less than one week before procedure, patient with significant LV dysfunction, 35% LVEF or less, patient with cancer or other severe chronic disease with life expectancy of ≤2 years, patient with chronic renal failure with serum creatinine ≥ 2 mg/dL, hemoglobin ≤11 g/dL, patient known or suspected not to tolerate the contrast agent, morbid obesity (BMI >40), drug abuse or alcoholism, patients under custodial care, pregnant women or women with childbearing potential with a positive pregnancy test at the time of procedure, were not enrolled into the study. The study was approved by the local Ethics Review Committee. Written informed consent was obtained from all patients prior to any study related activities.
In an initial pilot study, three patients with coronary CTOs were treated with the first generation device followed by device modifications leading to the development of the new CiTop™ Expander™ guidewire.
Study protocol
It was a prospective, first-in-man, single centre, open-label and non-randomised study to evaluate the safety and efficacy of the CiTop™ Expander™ guidewire for crossing chronic total occlusion in coronary arteries. Baseline demographics, haemodynamic, biochemical and pathological parameters as well as electrocardiographic, echocardiographic and angiographic characteristics were recorded. PCI was performed according to the conventional methods. Successful crossing of CTO by placement of CiTop™ Expander™ distal to occlusion with no device related major complications determined the primary endpoint. Device related complications, such as perforation and dissection of arterial wall, bleeding and cerebrovascular accident, occurrence of slow/no reflow and usage of intracoronary vasodilators like adenosine, sodium nitroprusside, nitroglycerine and GP IIb/IIIa receptor antagonists were recorded. TIMI flow and myocardial blush grade (MBG) pre- and post-PCI were assessed and the total fluoroscopy time and time required to cross the occlusion using Ovalum CiTop™ Expander™ was recorded. Patients were followed on 7th day and 30th day for major adverse cardiac events.
Device description
The CiTop™ ExPander™ guidewire is a 0.014” guidewire which combines conventional guidewire navigational properties with a tip dilation feature to allow its advancement through difficult occlusions. The CiTop™ ExPander™ guidewire is identical to Ovalum’s CE-approved and FDA cleared CiTop™ 6 CoBra™ peripheral guidewire in all aspects of design, materials and method-of-use. The only difference is its indication for use, which has been extended to its utilisation also in the coronary arteries. The CiTop™ ExPander™ guidewire consists of the following elements: 0.014” guidewire, working Element (WE), operator handle control mechanism, and an extension wire (Figure 1). The CiTop™ ExPander™ guidewire body is a 160 cm long 0.014” in diameter wire with a dilatable tip. The wire can be extended to 300 cm by connecting an extension wire to the tapered proximal end of the guidewire. The working element (WE) is a nitinol wire at the tip of the guidewire that can form an arc via the operator handle (Figure 2). The control handle is the operator’s control system. The handle is connected at the proximal end of the guidewire and provides the operator control over tip dilation, torquing and advancement. By pressing the handle button, the operator dilates the tip of the guidewire. A 140 cm extension wire is supplied with the CiTop™ ExPander™ guidewire.

Figure 1. CiTop™ ExPander™ guidewire elements.
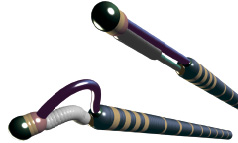
Figure 2. Illustration of the guidewire tip.
Procedure description
The study participants who qualified all eligibility criteria underwent standard angiography and CiTop™ Expander™ guidewire placing attempt. Patients were loaded with aspirin ≥150 mg and clopidogrel 300-600 mg dose before the procedure. Baseline biochemistry and pathology parameters were estimated. All equipments to be used during the procedure were carefully examined prior to angioplasty to verify their proper functioning. Before placing the CiTop™ Expander™ wire, the handle button was operated outside the body while verifying proper tip expansion according to fig. 2 and 3. The handle was rotated and the verified tip rotated accordingly. The CiTop™ Expander™ guidewire was used in a conventional manner as a 0.014” guidewire, using angiography to observe and monitor its path. The guidewire was introduced directly into guiding catheter to the target occluded lesion. The guidewire tip was navigated to the occlusion site as in standard guidewire practice and then inserted slowly into the occlusion proximal cap using conventional methods. Once the penetration of distal tip was accomplished, the guidewire could be manipulated to cross the CTO as a standard wire or by pressing the handle and dilating the tip. When the crossover had been accomplished, the control handle could be removed by opening the two torquer caps on its distal and proximal ends. The guidewire could be left in place to be used with conventional balloon catheters and stents or replaced by floppy guidewire using a microcatheter. This was followed by a balloon and stent implantation, according to the operator’s discretion. The use of lytic therapy or GPIIb/IIIa inhibitors was made at the cardiologist’s discretion. Post-PCI aspirin ≥100 mg orally indefinitely and clopidogrel 75 mg for at least 30 days and continued as per the physician’s discretion (as per ACC/AHA/ESC guidelines).
Study endpoint
Successful crossing of CTO by placement of CiTop™ Expander™ distal to occlusion with no device related major complications was the primary endpoint of the study. Secondary endpoint was successful stenting, procedure duration from insertion until crossing, fluoroscopy time, amount of contrast, lack of procedure related death, stroke, AMI, and no mechanical damage to the device during the crossing attempts.
Definitions
Chronic total occlusion was defined as complete obstruction of a native coronary artery with no luminal continuity and with TIMI flow grade 0. For PCI, procedural success was defined as restoration of TIMI flow grade 3 with a residual stenosis of <30% in target CTO lesion after stent implantation. Clinical success was defined as procedural success in the target CTO lesion without major adverse cardiac events (MACE). MACE was defined as a composite of death, non-fatal MI or urgent target vessel revascularisation. Technical success was defined as angiographic success (<20% residual stenosis and TIMI flow grade 3). Clinical success was defined as procedural success without death, (re)infarction (Q- and non–Q wave), emergent bypass surgery, target lesion revascularisation (coronary artery bypass surgery (CABG), or repeats percutaneous transluminal coronary angioplasty (PTCA), and cerebrovascular accidents (CVA: disabling stroke) and cardiac tamponade during the hospital stay. Safety was evaluated by the occurrence of bleeding defined as a drop of Hb of >5 mg/dl or requiring transfusion of four or more units of red blood cells; perforation of the vessel wall defined as leakage of contrast dye into the pericardial space, and significant dissection of the vessel. Major adverse cardiac events include freedom from cardiac death, (re)infarction (Q- and non–Q wave), emergent bypass surgery, target lesion revascularisation, and disabling stroke. A positive diagnosis of myocardial infarction was made if the following criterion of a typical rise and fall of biochemical markers of myocardial necrosis (including troponin, CK-MB, and CK) to greater than 3×ULN (or if markers already elevated, greater than 50% of the lowest recovery enzyme level from the index infarction) with at least one of the following: 1) ischaemic symptoms; 2) development of pathological Q waves on the ECG; 3) ECG changes indicative of ischaemia (ST segment elevation or depression).
Statistical analysis
Continuous variables are presented as means ± standard deviation and categorical variables are presented as frequencies (%).
Results
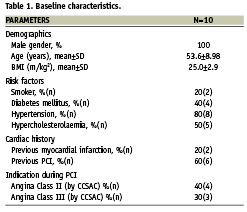
Ten patients suffering from chronic total occlusion suitable for percutaneous coronary intervention were included in the study. Baseline characteristics such as demographics, risk factors, cardiac history and haemodynamic parameters, biochemistry and pathology findings were recorded in Table 1.
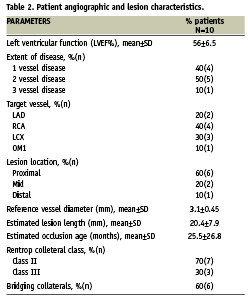
Angiographic and lesion characteristics
Mean ejection fraction (LVEF) was 56%±6.5. Most patients included in the study had double vessel disease (50%) or single vessel disease (40%). Right coronary artery (RCA) was the culprit coronary vessel in 40% and left circumflex (LCX) in 30% patients with type C lesion in 100%. Most of the patients (60%) had lesion location in proximal part of the coronary artery. The mean (±SD) occlusion diameter and length was 3.1±0.45mm and 20.4±7.9mm, respectively. Estimated mean (±SD) occlusion age was 25.5±26.8 months, with 60% patients having bridging collaterals (Table 2).
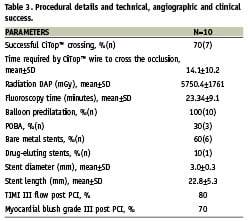
Procedural details including technical, angiographic and clinical success
Procedural success was achieved in seven (70%) of the patients. The total mean actual time required by the wire to cross the occlusion was 14.1±10.2 min. Following the successful crossing of the Ovalum CiTop™ Expander™ predilatation was performed in all the patients; most of the lesions were stented utilising bare metal stents (90%). The mean stent length and diameter implanted was 22.8±5.3 mm and 3.0±0.3 mm, respectively. Final mean TIMI flow and myocardial blush grade were 2.9±0.33 and 2.6±1.0, respectively. The mean total fluoro time and radiation exposure was 23.34±9.1 min and 5750.4±1761 mGy, respectively (Table 3). Clinical success was obtained in seven (70%) of the patients. In the three cases of device failure, two cases were due to inability to advance the CiTop™ Expander™ inside the occlusion and one case due to inability to navigate the CiTop™ Expander™ to the occlusion. In these three cases, a second attempt was made using a different CTO dedicated guidewire which caused a type A dissection in two cases and in all CTO was not revascularised without MACE.
Discussion
The Ovalum CiTop™ Expander™ guidewire was found to be safe and efficacious in recanalisation of coronary CTOs. The rate of procedural and clinical success was 70%.
Procedural success rates of percutaneous intervention for CTOs have increased significantly as a function of improved guidewires and devices, as well as operator technique and experience, such that successful recanalisation of true CTOs may now be achieved more frequently2,3. Even in experienced hands, the commonest reason for failure is inability to cross the CTO with 0.014’’ guidewire. In recent data from several experienced centres, the success rate of CTO revascularisation is between 51 to 73%6-9. The Mayo Clinic investigators showed an increase in the success rate of CTO revascularisation from 51% with angioplasty alone to 70% in the drug eluting stents era9. However, this success rate cannot be translated to all centres, and new specialised, easy-to-use, devices are required.
At the present time, different approaches are used for CTO recanalisation including: dedicated guidewires, ablative devices, mechanical devices and specialised techniques such as re-entry and retrograde approaches10. Ovalum’s CTO solution, the CiTop™ ExPander™ guidewire, enables the average interventional cardiologist to cross over the arterial blockage in a controllable and reproducible way. The system is made of a highly flexible guidewire body and a distal tip consisting of a micro-channel dilating working element.
The CiTop™ ExPander™ guidewire is similar in size and shape to the standard vascular guidewires, which received the CE Mark and FDA approval several years ago and are being sold by various companies in Europe, USA, as well as in many other countries. The CiTop™ ExPander™ guidewire is made of approved biocompatible materials and contains the metallic components like nitinol core wire; platinum springs for radio opacity detection, and stainless steel. CiTop™ ExPander™ guidewire is compatible with all standard PCI equipment and should be manipulated as a standard guidewire for delivering balloon catheters and stent delivery systems to the target lesion once the occlusion is crossed over. The features of the Ovalum CiTop™ Expander™ guidewire in the present first-in-man study as assessed by the investigator received excellent trackability, torqueability, trapability, penetrability, stability, shaft support, probing force, radio opacity, and manoeuvrability scores. The compatibility with other cathlab equipment like deliverability of balloon over the CiTop™ Expander™ guidewire, deliverability of stent over the CiTop™ Expander™ guidewire, deliverability of balloon across the lesion over guidewire and deliverability of stent across the lesion over the guidewire was very easy. The working element of the CiTop guidewire, which creates a blunt micro-dissection takes advantage of the elastic properties of the arterial adventitial layer allowing the device to dissect bluntly without the potential of perforation.
During the study period, no major adverse cardiac events were reported using the Ovalum CiTop™ ExPander™ guidewire. These preliminary findings need to be confirmed in a large multicentre, prospective trial with large number of patients.
The currently available dedicated CTO device with similarities to the CiTop™ ExPander™ guidewire includes the Frontrunner (Lumend) catheter which is a manually operated device with a hinged tip which uses a form of blunt micro-dissection to cross the lesion. However, this is a bulky device which is now oriented to treat peripheral CTOs where the risk of coronary perforation was relatively high11. The Safe-Cross wire, guided by radiofrequency, total occlusion crossing system in chronic coronary total occlusions reported similar success rates, but there was a perforation rate of 0.9%12. The Crosser System™ is also a new dedicated CTO device. This device consists of a monorail catheter delivering vibrational energy to facilitate CTO crossing. The safety and increased success of opening CTO refractory to guidewires has been discussed13. A recent report showed that CROSSER-assisted guidewire recanalisation was achieved in 60.8% of refractory CTOs14.
This first-in-man report is limited by several issues. First, this experienced is based on only 10 patients in a single centre. Second, there might be a learning curve until it can be optimally operated by an average interventional cardiologist. However, we are encouraged by the easy-to-use properties of the device and the compatibilities with regular coronary balloons and stents.
In conclusion, in this first-in-man study, the Ovalum CiTop™ Expander™ guidewire seems to be safe and efficacious in recanalisation of coronary CTOs. The CiTopTM Expander ™ may be routinely used as a first line device for crossing CTOs. Further studies will be required to support these encouraging results.
List of investigators
Dr. Amit Segev, The Heart Institute, Tel-Hashomer, Israel; Dr. Keyur H. Parikh, The Heart Care Clinic, Ahmedabad, India; Dr. Milan C. Chag The Heart Care Clinic, Ahmedabad, India; Dr. Urmil G. Shah, The Heart Care Clinic, Ahmedabad, India; Dr. Hemang A Baxi, The Heart Care Clinic, Ahmedabad, India; Dr. Anish H Chandarana The Heart Care Clinic, Ahmedabad, India; Dr. Ajay M. Naik, The Heart Care Clinic, Ahmedabad, India; Dr. Joyal N Shah, The Heart Care Clinic, Ahmedabad, India; Dr. Mihir P. Tanna, The Heart Care Clinic, Ahmedabad, India; Dr. Jayesh Bhanushali, The Heart Care Clinic, Ahmedabad, India; Dr. Ravi S. Singhvie, The Heart Care Clinic, Ahmedabad, India.

