Abstract
Aims: The major challenge for the interventional treatment of chronic total coronary occlusion (CTO) is a low primary success rate. A common problem is the passage of the recanalisation wire into a subintimal position. New devices, which were evaluated in the first multicentre study in CTOs resistant to a conventional wire approach, may help to facilitate a controlled re-entry into the true lumen. The aim of this study was to assess the safety and efficacy of this approach, with successful true lumen distal wire passage as the primary endpoint.
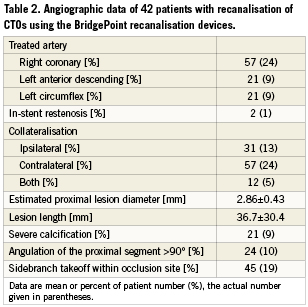
Methods and results: Forty-two patients were enrolled in four centres with high expertise in PCI for CTOs. All CTOs were of at least three months duration, and were initially attempted with dedicated recanalisation wires. After failure to pass or creation of a subintimal dissection, the BridgePoint devices were applied, consisting of a ball-tipped catheter (CrossBoss) to pass the proximal occlusion cap, and a flat-shaped balloon catheter (Stingray catheter) to be inflated within the subintimal space to guide the re-entry into the true vessel lumen with a special wire (Stingray guidewire). The primary endpoint was met in 67% of all patients. A higher success rate seemed to be possible when all devices were used in sequenced beginning with the CrossBoss, and in the case of a subintimal passage, followed by the Stingray. True lumen re-entry failed because of the loss of distally contrast filling and thus loss of a target for re-entry, and by a failure to advance the Stingray balloon far enough distal and parallel to the distal lumen. There were no severe device related complications.
Conclusions: In patients with complex CTOs referred to dedicated centres with high experience in CTOs, these results demonstrate the potential of a guided re-entry from a subintimal wire position by use of the BridgePoint devices.
Introduction
The rationale for recanalising a chronic total coronary occlusion (CTO) is the relief of angina pectoris1, the improvement of an impaired left ventricular (LV) function2-5, to avoid CABG and a favourable effect on survival6,7. The percutaneous coronary intervention (PCI) in CTOs is hampered by two major problems, 1) a low success rate8, and 2) a higher rate of target vessel failure than in non-occlusive lesions. The use of drug-eluting coronary stents (DES) in CTOs overcame the second limitation9-11, whereas the low success rate remains a major setback in the routine approach to CTOs.
The main reason for a procedural failure is the inability to cross the occlusion with the guidewire into the distal true lumen, frequently ending up in the subintimal space12. This had been documented by intravascular ultrasound13. A re-entry into the true lumen from the subintimal space can be achieved either by a forced subintimal tear with a wire, as described in the so called STAR technique (subintimal tear and re-entry) but the site of re-entry is not controlled, and side branches may be sheared off14,15, or by re-puncturing the true lumen with a stiff-tipped wire – quite difficult and often unsuccessful. In the treatment of peripheral arterial occlusions of the superficial femoral artery, the same problem is faced and overcome by a guided penetration of the subintimal layer towards the true lumen with a needle16,17. In the smaller dimension of the coronary arteries, no such dedicated device had become available until today.
The BridgePoint devices (BridgePoint Medical Inc., Plymouth, MN, USA) consist of a blunt tipped catheter to either pass the occlusion or at least create a subintimal entry (CrossBoss), a flat shaped balloon with side exit holes (Stingray catheter), and the appropriate small diameter wire with an angled and sharpened tip (Stingray guidewire) to exit from these holes and re-enter the true lumen, are the first set of tools specifically designed to facilitate the controlled subintimal re-entry into the true lumen distal to a coronary occlusion. After an extensive series of in vitro evaluations, and the first in man application to test this approach, this multicentre study was planned to show the applicability of the devices in complex CTO cases treated by dedicated experts.
Methods
Study protocol
The primary objective of the study was to test the ability of the CrossBoss and Stingray catheters as well as the Stingray guidewire to facilitate safe and effective intra-luminal placement of a guidewire beyond coronary CTOs without a significant increase in major acute complications as compared to conventional guidewire / support catheter technology. This was a prospective, non-randomised multicentre study in patients with coronary artery CTOs who were scheduled to undergo an interventional procedure using a dedicated CTO guidewire and catheter equipment and technique.
The refractory nature of these patients was established and documented through an attempt to cross the CTO using dedicated recanalisation guidewire(s), typically from the ASAHI family MiracleBros and Confianza (ASAHI Intecc, Aichi, Japan). A best effort attempt to place a conventional guidewire in the true vessel lumen distal to a CTO should be done with a minimum of 20 minutes of procedural time. Any patients with CTOs successfully crossed within this time were excluded from the study.
The refractory nature of these patients was also established and documented where the best effort attempts results in recanalisation guidewire advancement into the subintimal space. In these cases, the Stingray catheter could be immediately used (within the 20 minute best effort period) in an effort to establish a pathway back into the true vessel lumen, and thus further facilitate the placement of a guidewire across the CTO lesion. In these patients, techniques that aid in the advancement of the Stingray over the guidewire may be used (for example, buddy wire, dilatation with a standard balloon, Tornus, CrossBoss, etc.)
Once the refractory nature of the CTO had been confirmed, the BridgePoint Medical CrossBoss catheter could be introduced and used to attempt to cross the CTO.
In cases where the use of the CrossBoss and Stingray devices made it possible to place a guidewire across the CTO in the true vessel lumen, appropriate PTCA / stent therapy would then be separately undertaken as therapeutic treatment. The ultimate therapeutic outcome associated with the use of angioplasty devices was not the subject of this study, but is reported. However, given the difficulty of definitive differentiation of MACE rates between the use of PTCA, stent and initial crossing (CrossBoss and Stingray) technologies, 30 day major cardiac adverse event rates (MACE) were evaluated.
Device description
The CrossBoss™ Catheter (Figure 1) is a single use, over-the-wire, disposable, percutaneous catheter designed for facilitating crossing of occlusions in the coronary vasculature. This is achieved by steering the 1 mm diameter distal tip of the device to the occlusion site within the native blood vessel lumen and using the fast-spin technique to advance the catheter as necessary past the occluded lesion. The distal portion of the CrossBoss is hydrophilic coated to enhance lubricity. A torque device, coaxially positioned over the proximal portion of the CrossBoss catheter, provides a comfortable user interface for device manipulation and includes a clutch that prevents over-torqueing that might cause damage to the device or the vessel. The device delivers a guidewire beyond the diseased segment. Subsequent to conventional guidewire placement, PTCA catheters and stents may be used to provide therapeutic benefit. The BridgePoint Medical CrossBoss device in and of itself does not provide any therapeutic effect or benefit beyond simple facilitation of guidewire crossing.
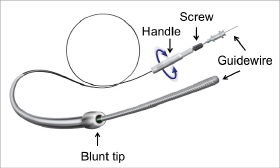
Figure 1. The CrossBoss catheter features a blunt tip that is rotated by manual rotation of the proximal handle with a guidewire inserted (which is not advanced out of the distal central lumen during catheter advancement). The torque device is adjustable on the shaft by a screw, and prevents over-torquing.
The Stingray® catheter is a sterile, single use, disposable catheter designed to further facilitate placement of a guidewire across a CTO lesion by enabling re-entry into the true vessel lumen in cases where a conventional guidewire or the CrossBoss has been advanced into the subintimal plane (Figure 2). This is accomplished through the use of a distal inflatable element, that when inflated with radiopaque contrast media provides visibility and intra-arterial stability. The distal inflatable element consists of two small calibre inflatable balloons positioned adjacent to a guidewire lumen. When inflated, these small calibre balloons along with the lumen generally define a planar geometry where the width of the construction is approximately 2.5 times its height (2.5 mm and 1 mm, respectively). Within the inflatable element, the distal portion of the catheter includes two oppositely facing lateral ports that communicate with the central guidewire lumen. These ports allow the operator to direct a guidewire from the wire lumen at an angle to the catheter shaft. The inflatable element along with the lateral ports make it possible to accurately position the distal tip of a guidewire in such a way as to allow entry back through the internal subintimal tissue without harm to the adventitia and external vessel wall. In this manner the device provides a pathway for advancement of a guidewire back into the true vessel lumen distal to the CTO.
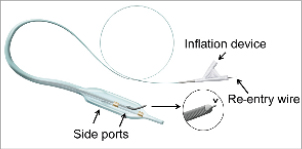
Figure 2. The Stingray balloon catheter is an over-the-wire design which features the flat distal balloon with a distal wire lumen and two side ports that are connected to the central lumen. The Stingray re-entry wire is inserted and can be directed because of its distal angle through the side ports. The wire features a distal needle-like tip (arrow head in the insert) to facilitate the penetration from the subintimal space into the true lumen.
The Stingray guidewire (Figure 2) is a single-use, disposable, 0.014” diameter guidewire designed to enhance guidewire re-entry into the true vessel lumen during the use of the Stingray catheter. The distal portion also includes a platinum coil for visibly. The distal tip of the guidewire includes an angled geometry with a 28 degree bend in the distal 1.5 mm section. The distal diameter is 0.25mm. The probe extends from the distal tip 0.18 mm and has a 0.09mm diameter to help facilitate re-entry back through the internal subintimal tissue without harm to the adventitia and external vessel wall. The tip load is similar to a Confianza 9-12 gram wire.
Patients
Between July 2008 and June 2009 a recanalisation of a CTO of proven duration of more than three months in a major coronary branch (diameter >2.0 mm) was attempted in 42 patients. The patient inclusion required the presence of sponsor personnel; therefore, the inclusion was not consecutive and represented about 15% of patients with CTO treated during that time period. The indication for revascularisation was either chest pain or persistent occlusion after a prior myocardial infarction (MI) and documented viability. Written informed consent was obtained from all patients. The study had been approved by the local regional ethics committee.
Angioplasty procedure
All patients successfully recanalised were on aspirin (100 mg) and received clopidogrel (75mg) with a loading dose of 600 mg, for 12 months starting on the day of the procedure. A femoral approach was chosen with 7-8 Fr guiding catheters. The initial attempt was to cross the lesion with dedicated guidewires supported by an exchange catheter or a low profile over-the-wire balloon catheter. This conventional wire approach was stopped if it did not lead to an advancement of the wire beyond the proximal occlusion cap within 20 minutes, or if the wire entered the subintimal space. Wire advancement was monitored by contrast filling of the distal vascular bed via the collaterals. This was observed through ipsilateral collaterals in 31% of patients. In the patients with only contralateral filling of the distal vascular bed (57%) or both ipsilateral and contralateral filling (12%), an injection through the contralateral coronary artery was done using a 4 Fr or 5 Fr diagnostic catheters through a second femoral sheath.
The balloon dilatation was performed sequentially with increasing sizes, and the appropriate stent size was selected after i.c. nitroglycerine. The procedural strategy was to cover the occlusion site completely with one or more stents of the available lengths, without leaving a dissection or a residual stenosis of >15% in the adjacent segments. In case of multiple stents minimal overlapping was assured. A balloon-to-artery ratio of 1.1 with inflation pressures of 12-16 atm was used.
Angiographic analysis
The angiographic features of the occlusion were assessed at the beginning of the procedure. The length of the occlusion was assessed as the lesion length visible on simultaneous injection into the proximal and the collateralised distal segment of the CTO. The LV function was analysed using a standard software program incorporated in the coronary angiography system.
Study endpoints
Success was defined as the ability of the CrossBoss and Stingray catheters along with conventional and the Stingray guidewires to facilitate the placement of a guidewire across the CTO in the true vessel lumen without an increase in acute complications (associated with the use of the CrossBoss or Stingray devices) as compared with guidewire adverse event rates reported in the literature, and overall one month MACE (including cardiac death, lesion-related AMI and emergency bypass surgery involving the treated segment) rates published in the literature8,18,19.
Statistics
Data are given as mean ± SD or percentage. Group differences of continuous variables were evaluated by a t-test. Group differences of categorical variables were tested by a X2-test. A level of p<0.05 was considered significant. All calculations were done with SPSS for Windows, Version 16 (SPSS Inc., Chicago, IL, USA).
Results
Patient and procedural characteristics
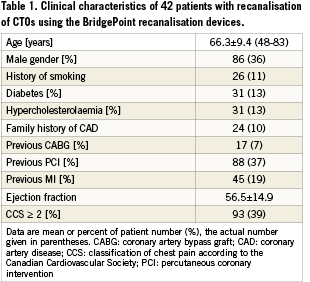
The new set of devices by BridgePoint for a controlled antegrade tracking and subintimal re-entry were applied during recanalisation of CTOs by four dedicated operators with high experience in PCI of CTOs. The selection of cases was not limited to specific types of CTOs, however, ostial occlusions were excluded. The indication for the PCI was based on clinical symptoms or ischaemia caused by the CTO. The group of 42 patients included in this study was a subset of 417 patients treated in the participating centres during the study period in whom the initial wire approach failed to cross the occlusion within at least 20 minutes, or where a subintimal wire position was observed during the course of the procedure. The success rate in the participating centres ranged from 83-88%, but the study patients represented a negative selection. The patient characteristics are summarised in Table 1.
The complexity of the lesions attempted with the BridgePoint devices was high as shown by the lesion length of 37 mm on average (Table 2). More than half of the lesions were located in the right coronary artery. About half of the cases had good contralateral collaterals and would have been candidates for a retrograde attempt in case of failure. Calcifications and severe angulation were found in only about 20%, respectively, but side branch take-off at the site of the occlusion was frequent with a 45% occurrence.
Procedural outcome
The procedural flow of the use of the BridgePoint devices is shown in Figure 3. Technical success was achieved in 67% of all cases followed by successful stent deployment and reestablishment of TIMI 3 flow in all cases, providing also procedural success in 67%. The wire attempt with dedicated recanalisation wires before switching to the BridgePoint devices took 19 minutes on average, but in five cases this occurred only after extensive attempts of more than 30 to 90 minutes. As these new devices required proctoring and technical advice, the first two cases of each operator were done with the presence of proctors, and in these cases the cross-over from the conventional wire approach to the use of the BridgePoint devices was less than 20 minutes of refractory time.
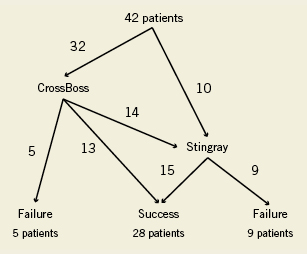
Figure 3. Flow chart of the study progress.
There was a trend to an earlier switch from the conventional approach to the BridgePoint device use in successful cases, but this was not significant (successful: 9±12 min vs. unsuccessful: 17±24min). The procedure time overall was 110 minutes on average, and the fluoroscopic time 41 minutes on average (Table 3). There was no relevant difference in these times if either the CrossBoss was used alone, or in conjunction with the Stingray balloon, or if the Stingray balloon was attempted alone after subintimal wire passage. The success rate for using the CrossBoss and Stingray in sequence tended to be higher with 71% as compared to using the Stingray as bailout after subintimal wire passage with 50% success. A case example of a patient treated with the full sequence of devices is shown in Figure 4.
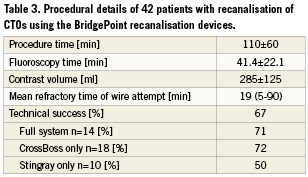
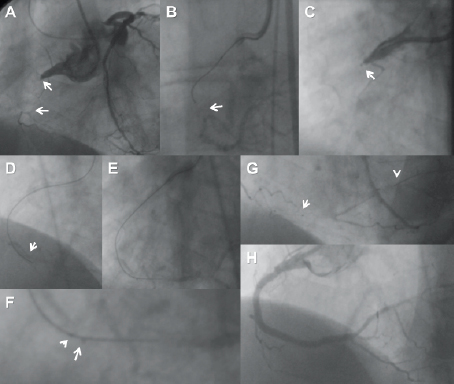
Figure 4. Patient (68, male) with a 20 mm long tapered occlusion of the right coronary artery (A), the distal end of the occlusion is positioned at a small side branch (arrow). (B): Failed attempt with a Miracle 3 wire (ASAHI Intecc, Aichi, Japan) which deviates from the true lumen (arrow). (C): A CrossBoss catheter is advanced into the proximal cap (arrow), then the Miracle re-advanced distally, followed further by the CrossBoss. (D): The wire is past the distal true lumen (arrow head). (E): Then the Stingray balloon is exchanged over the wire and advanced. The Stingray re-entry wire is in place and already advanced outside of the distal side hole of the Stingray balloon. The magnification of the distal balloon tip (F) shows the two radio-opaque markers (arrow heads) of the Stingray balloon. The first side hole is located proximal to the proximal marker, the second side hole between the proximal and distal marker. The wire exits between the markers towards the inner curvature of the vessel which is recognised by the passage sideways of the distal marker. The wire enters the distal vessel lumen which is verified by the contralateral injection with the wire tip inside the contrast filled lumen (arrows in E and F). (G): The Stingray balloon is first pulled back over the wire, then a microcatheter advanced (arrow: QuickCross [Spectranetics, Colorado Springs, CO, USA]), and then the stiff re-entry wire exchanged for a floppy wire (arrow head). The final result after placement of three drug-eluting stents (XienceV [Abbott Vascular, Redwood City, CA, USA ]) is shown in (H).
In the one case of in-stent reocclusion located in the mid LAD the CrossBoss catheter was successfully applied to cross the occlusion and reached the distal true lumen without subintimal passage.
Patients in whom the procedure failed were brought back at a later date to perform a second attempt whenever it appeared feasible, with the possibility to use a retrograde approach at that later time point. It was not intended to switch to retrograde within the study procedure.
Modes of failure
In 18 cases in which the CrossBoss catheter was used to cross the proximal cap, the procedure failed in five cases because of the inability to cross the cap. In the remaining 13 cases the CrossBoss catheter gained the distal true lumen and a wire could be advanced intraluminally to the distal vessel segment. In 24 cases the wire went subintimal either after preparation of the lesion with CrossBoss catheter (14 cases) or after the initially used guidewires had passed the proximal cap and then went subintimal (10 cases). In the former situation after preparing the lesion with the CrossBoss, the Stingray balloon could not be advanced to a proper position where a re-entry puncture could be attempted in two cases, and in two cases the re-entry Stingray wire could not be directed into the distal true lumen due to loss of contrast filling of the distal target. In the 10 cases where a re-entry was attempted after wire advancement, the Stingray balloon could not be advanced in three cases, and the re-entry puncture failed because of loss of distal contrast filling in two cases.
Procedural safety
There were no penetrations outside of the adventitia with either the CrossBoss catheter or the Stingray balloon and Stingray re-entry wire. The only major adverse event within 30 days was the incidence of periprocedural non ST elevation myocardial infarction (Table 4). This occurred in two patients after successful recanalisation most probably due to the occlusion of a side branch after stent placement. Both patients were asymptomatic, and the enzyme rise was short lasting with a peak within 24 hours after the procedure. Both patients were discharged without further sequelae.
Discussion
The problem zones of a CTO for wire crossing
The process of recanalising a CTO involves three major steps, the penetration of the proximal occlusion cap, the advancement of the wire within the occlusion, where we often find loose tissue of organised thrombus, and the re-entry through a distal cap into true lumen of the distal vessel segment20. Penetration of the proximal cap is often a matter of perforation force, which had been increased by technical advancements in coronary guidewire construction, and only severely calcified lesions may still be resistant to a wire passage18,19.
Even with correct puncture of the proximal cap, during further advancement of the wire within the occlusion with its often mixed softer and harder material, as illustrated by histological and intravascular ultrasound investigations20-22, stiff but also lubricious wires may deviate into the subintimal space as there is no way of visualisation of the wire position relative to the lumen in these segments. Finally, the distal exit point of the occlusion where the wire aims at an often collapsed distal vessel lumen carries another risk of subintimal deviation.
Subintimal re-entry
Different strategies to achieve a re-entry had been developed such as a brute force advancement of a wire loop with the aim to gain distal re-entry, but with the high risk of shearing off possibly important side branches (STAR-technique)14,15. A more controlled approach are the attempt to re-enter with stiff and tapered wires with or without the assistance of IVUS – a method with limited success, or variations of the CART technique23-26, requiring a retrograde wire passage through collateral channels to be combined with an antegrade wire advancement, and with either proximal or distal (retrograde) advancement of balloon catheters and dilatation to create local dissections to facilitate a wire re-entry into the true lumen. Especially the combined antegrade and retrograde procedures require long procedure times, and increased radiation.
Guided subintimal re-entry using the Stingray catheter and re-entry wire
In the present study, a guided re-entry from a subintimal wire position was tried in 24 cases either after a subintimal passage of a dedicated recanalisation guidewire, or after the application of the CrossBoss device for a blunt passage of the proximal cap. The re-entry into the distal true lumen with the Stingray re-entry wire from the subintimally advanced Stingray balloon catheter was achieved in 63% of cases, followed by a successful balloon dilatation and stent placement. The major cause of a failed re-entry was the loss of distal contrast filling because of extension of the subintimal space by a dissection and compression of the distal true lumen. Another problem was a failure to direct the re-entry wire towards a very small distal target lumen. It appeared during the progress of this study, that a major determinant of success was also the ability to advance the Stingray balloon far enough parallel to the distal lumen to a vessel segment with minimal angulation.
In all cases with successful re-entry, the duration of the procedure was similar to that reported with the recent experience with the antegrade approach, but lower than expected, if the retrograde approach were used. Given the above mentioned limitations when the device did not achieve a re-entry, it appears a feasible alternative to the switch to a retrograde approach right away after subintimal dissection. Even in case of failure, the retrograde option is still possible, although with regard to procedure time and contrast load, a further attempt should be deferred for a second attempt.
The CrossBoss device for crossing the proximal cap
In its design the CrossBoss catheter resembles the ROTACS recanalisation wire with a blunt ball-type tip27. The advancement is achieved with fast manual rotation to reduce friction and enable the forward push into the proximal cap. This was tried in 32 cases with certain preferred morphological features of the proximal occlusion morphology. As the device is not steerable by itself, ideally it would work with an occlusion with a well-defined entry, blunt or tapered, but without a larger side branch. Furthermore, the rigid design to enable the transfer of push, limits the passage through tortuous segments proximal to the occlusion. In the absence of these limitations, the device may even be able to pass the occlusion from true lumen to true lumen as shown in 13 of 18 of cases (72%) without additional need for the Stingray balloon. As the CrossBoss catheter has an over-the-wire design, guidewires can be exchanged during the procedure. Most often a Miracle wire was used for the advancement of the device, and if the device could not be advanced with the wire pushed back inside, the wire could be used to find an initial pathway into the proximal cap to facilitate the device advancement. In case of an inability to further advance the ball tip a sort of limited STAR technique could be tried with a soft lubricious wire to enlarge the subintimal space (Craig Thompson, MD, personal communication), but this approach had not been applied systematically within this study.
The CrossBoss tip has a tendency to leave the true lumen into the subintimal space especially at vessel bends where it tends to be directed towards the outer curvature, but neither in this study nor in the earlier pilot phase of the device application did the ball tip ever exit the adventitia. So it appears safe with respect to vessel perforations, if every caution is administered to avoid side branch entry at the proximal entry into the occlusion. The creation of a subintimal space with the CrossBoss catheter is often sufficient to enable the advancement of the Stingray balloon, and thus, the combined use leads to a successful re-entry in 71% of cases.
Potential of the new device family
It is difficult to assess the potential of new devices for CTOs, as a major factor of the primary success rate is the operator’s experience and skill, as well as the inherent learning curve with a new device. Therefore, in this study the device success was assessed for only a small group of operators, but the inter-operator comparison already shows distinct differences most likely attributable to selection differences, as the overall success rate in CTO procedures among these operators was not in line with the success rate within this study. Furthermore, the learning curve is certainly relevant for such a new device approach, which is reflected by the current experience in the on-going US trial where a higher experience lead to higher success rates (Craig Thompson, personal communication).
Despite the influence of a learning curve, the technical feasibility and applicability of the device concept was proven in the current study of initially failed cases. As the operators were selected experienced physicians, it may be assumed that the devices were indeed used only after a thorough attempt with dedicated CTO wires (Miracle Bros 3 – 12G, Confianza Pro 9 – 12G [ASAHI Intecc]). The average refractory time was 19 minutes, but ranged up to 90 minutes, and dedicated recanalisation wires were used. In contrast to the current study, earlier studies of new devices required just 5-10 minutes of wire attempts with very soft wires which are not the standard choice of experienced CTO operators27-30. Despite these “easier” conditions, those devices showed lower success rates in less complex lesions than attempted with the BridgePoint devices in the present study.
Conclusions
From our initial experience, the CrossBoss catheter seems to be a valuable tool to facilitate the passage of the proximal cap, and may help also less experienced operators to achieve a CTO crossing with certain angiographic exclusion criteria like severe calcification, severe tortuosity of the proximal vessel, and absence of a proximal stump. The StingRay balloon catheter proved its potential of a guided re-entry, but the optimum use of the device requires proper and continued training and an experienced operator. These devices add a new procedural dimension to the toolbox of CTO operators, and offer an additional antegrade technical option especially in case of subintimal wire passage when the next step would be an IVUS guided re-entry or a retrograde strategy.
Conflict of interest statement
The authors have no conflicts of interest to declare.

