Abstract
Background: Equipment delivery in chronic total occlusion (CTO) percutaneous coronary intervention (PCI) can be challenging and it is associated with a higher risk of device entrapment. Data regarding the incidence of device entrapment during CTO PCI are lacking.
Aims: The aim of this study was to describe the incidence and procedural characteristics of device entrapment in patients undergoing PCI for CTOs and discuss management strategies for dealing with it.
Methods: Device entrapment was characterised in a large consecutive series of 2,361 CTO PCI cases performed by five high-volume CTO Italian operators between January 2015 and January 2020.
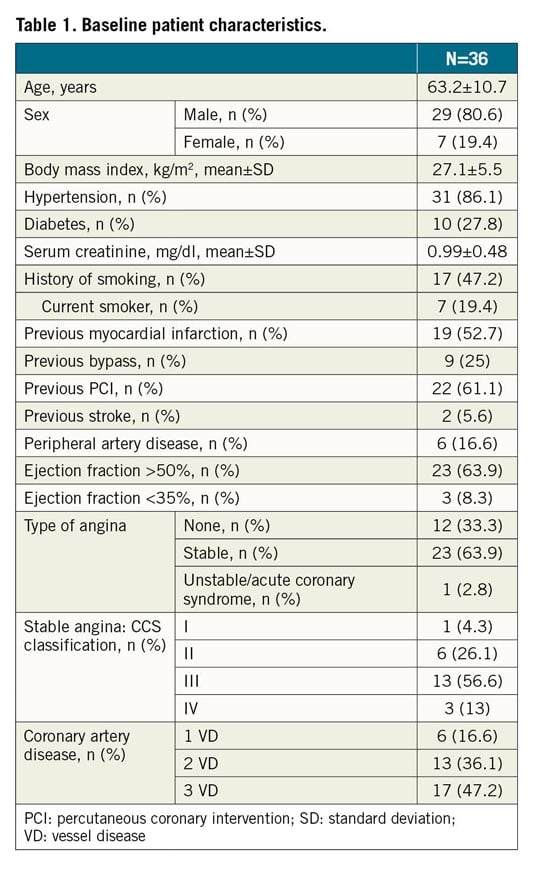
Results: Device entrapment occurred in 36 out of 2,361 cases (1.5%) and consisted of coronary guidewires in 13 (0.5%), microcatheters in 6 (0.2%), balloons in 6 (0.2%), rotational atherectomy burrs in 10 (0.4%) and guiding catheter extension in 1 patient (0.04%). Complete device retrieval was achieved in 63.9%, with at least partial removal of material in 97.2%. Vessel recanalisation was still possible in 86.1% of cases even after device entrapment. Intraprocedural myocardial infarction occurred in 3 patients (8.3%), tamponade with urgent pericardiocentesis in 1 (2.8%) and emergency surgical removal of the entrapped device in 1 patient (2.8%). Mean radiation dose was 4.7±2.3 Gy. At 30-day follow-up, one patient died with stent thrombosis of a non-target vessel and another required repeat percutaneous revascularisation.
Conclusions: Device entrapment during CTO revascularisations is a rare but potentially severe complication. We describe and discuss current techniques of percutaneous retrieval that can be employed to achieve procedural success safely.
Introduction
Chronic total occlusion (CTO) percutaneous coronary intervention (PCI) has rapidly evolved in recent years, with the development of novel techniques and equipment, leading to high success rates among experienced operators. However, CTO PCI still remains technically challenging and is associated with higher rates of procedural complications1. Equipment delivery in CTO PCI can be more challenging, due to the presence of severely calcified, tortuous vessels, and the need to negotiate very narrow channels, with a higher risk of device entrapment2. Data regarding the incidence of device entrapment during CTO PCI are lacking, with only anecdotal reports in the literature3,4,5. We collected a large series of CTO PCI to investigate the incidence, management strategies and clinical impact of device entrapment.
Methods
PATIENT POPULATION
We collected data on 2,361 consecutive CTO PCI procedures performed by five high-volume CTO operators (>100 cases per year) in Italy between January 2015 and January 2020 to identify cases of device entrapment. Data were retrospectively collected from each centre’s database. No exclusion criteria were applied. Information was obtained from medical records and angiograms.
STUDY DEFINITIONS
CTO was defined as a complete vessel occlusion with antegrade intraluminal Thrombolysis In Myocardial Infarction (TIMI) flow of 0 and clinical or angiographic evidence of occlusion duration ≥3 months. “Device entrapment” was defined as any material that, once delivered to the lesion site, got stuck in place and could not be retrieved with standard operational procedures (i.e., push-pull method), requiring any additional device (e.g., second wire, balloon, guide extension, snare). Retrieval success was defined as subsequent successful percutaneous extraction of the entrapped device(s) with further emergency dedicated techniques. Technical success was defined as the positioning of a guidewire in the distal true lumen of the attempted CTO, deployment of a balloon or stent with final antegrade TIMI flow grade 2 or 3, with residual stenosis <50% by angiographic evaluation and no significant side branch (>2 mm in diameter) occlusion. Major adverse cardiac and cerebrovascular events (MACCE) were defined as in-hospital death, procedure-related myocardial infarction (MI), emergent coronary artery bypass graft (CABG) or stroke. Procedural success was defined as technical success without MACCE. All deaths were considered of cardiac origin unless otherwise documented. MI was defined according to the Fourth Universal Definition of Myocardial Infarction6. Procedure time was defined as the time between local anaesthetic injection and removal of the last guide catheter. Target lesion revascularisation (TLR) and stent thrombosis were defined according to the Academic Research Consortium definitions7. One-month clinical follow-up was obtained by patient report during either an outpatient visit or a telephone call. This study complied with the Declaration of Helsinki and was approved by local ethics committees.
STATISTICAL ANALYSIS
Continuous variables are reported as mean±standard deviation (SD). Categorical variables are reported as N (%) and were compared using the chi-square test. Statistical analyses were performed using Stata, version 13.0 (StataCorp, College Station, TX, USA).
Results
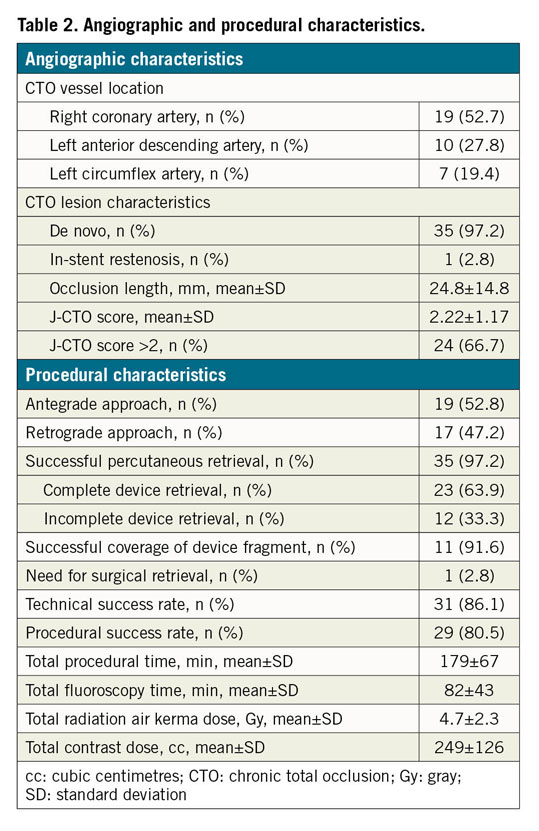
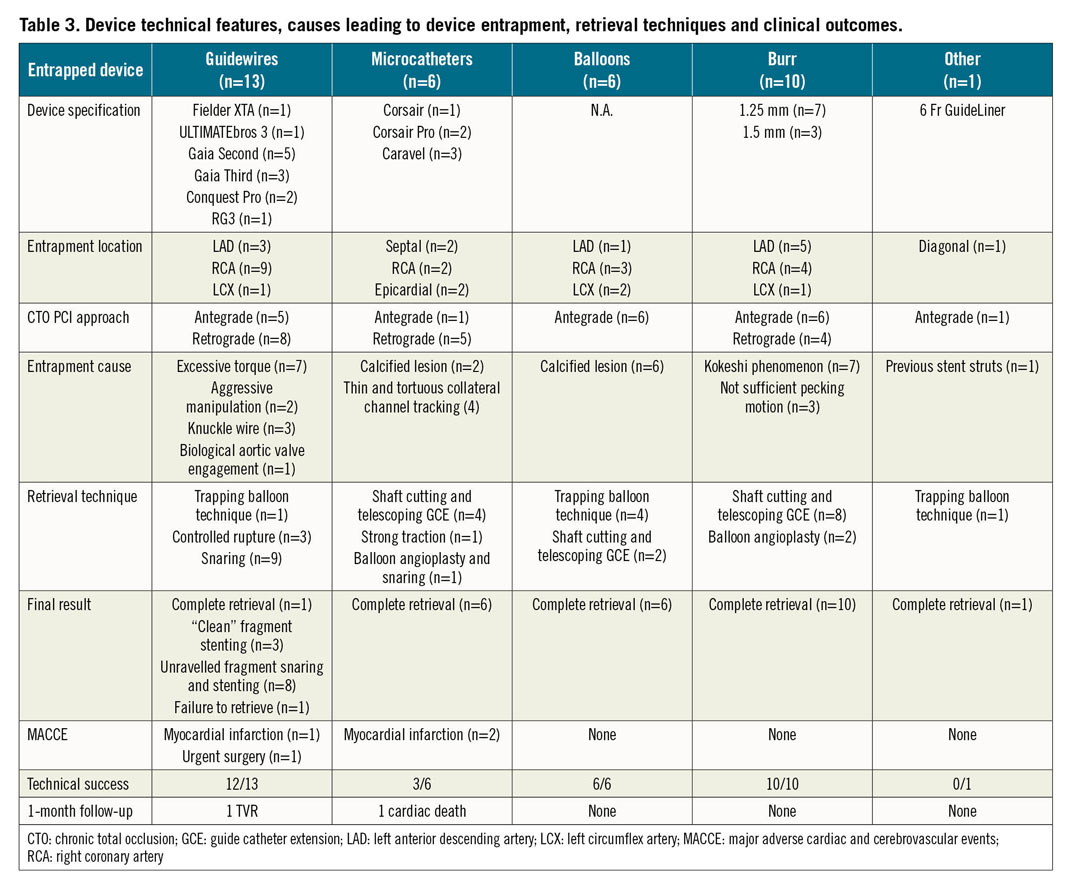
A total of 2,361 consecutive CTO PCI performed by five high-volume CTO operators in Italy were reviewed. The approach was antegrade in 1,486 cases (62.9%) and retrograde in 875 cases (37.1%). Device entrapment occurred in 36 cases (1.5%). Patient and angiographic characteristics are described in Table 1 and Table 2, respectively. Entrapped devices consisted of guidewires (n=13, 0.5% of cases), microcatheters (n=6, 0.2%), balloons (n=6, 0.2%), rotational atherectomy burrs (n=10, 0.4%) and one guide catheter extension (n=1; 0.04%). Mean J-CTO score was 2.22±1.17. Table 3 shows the types of device, causes leading to the entrapment, retrieval techniques and outcome. Nineteen cases of device entrapment occurred during antegrade CTO PCI and seventeen during a retrograde approach, with no significant differences between groups (1.3% vs 1.9%, p=0.203). The most common entrapped devices during antegrade CTO PCI were rotational atherectomy burrs (n=6) and balloons (n=6), while guidewires (n=8) were the most frequent entrapped devices during retrograde CTO PCI (Table 3).
DEVICE ENTRAPMENT MANAGEMENT
Management techniques of device entrapment are summarised in Figure 1 and Table 3. Percutaneous retrieval of the entrapped devices was achieved in 35 out of 36 cases (97.2%): complete retrieval was achieved in 23 (63.9%) cases, while partial retrieval of the entrapped material was achieved in 12 patients (33.3%). Among the 13 entrapped guidewires, one was completely retrieved using the trapping balloon technique. In three cases, a clean “controlled rupture” was achieved, and the guidewire’s tips were completely covered after stent implantation (Figure 2). In eight cases, retrieval attempts led to an unfolding of the tip coil into small filaments and a snare was required to remove the foreign body; residual unravelled fragments were successfully covered with a stent in seven patients. However, incomplete coverage of the unravelled segment occurred in one case due to the guidewire unravelling into a septal branch (Figure 3). Finally, one patient required emergent surgery due to failure to retrieve a retrograde RG3 wire (Asahi Intecc, Aichi, Japan) that became entrapped in a biological aortic valve prosthesis (Figure 4).
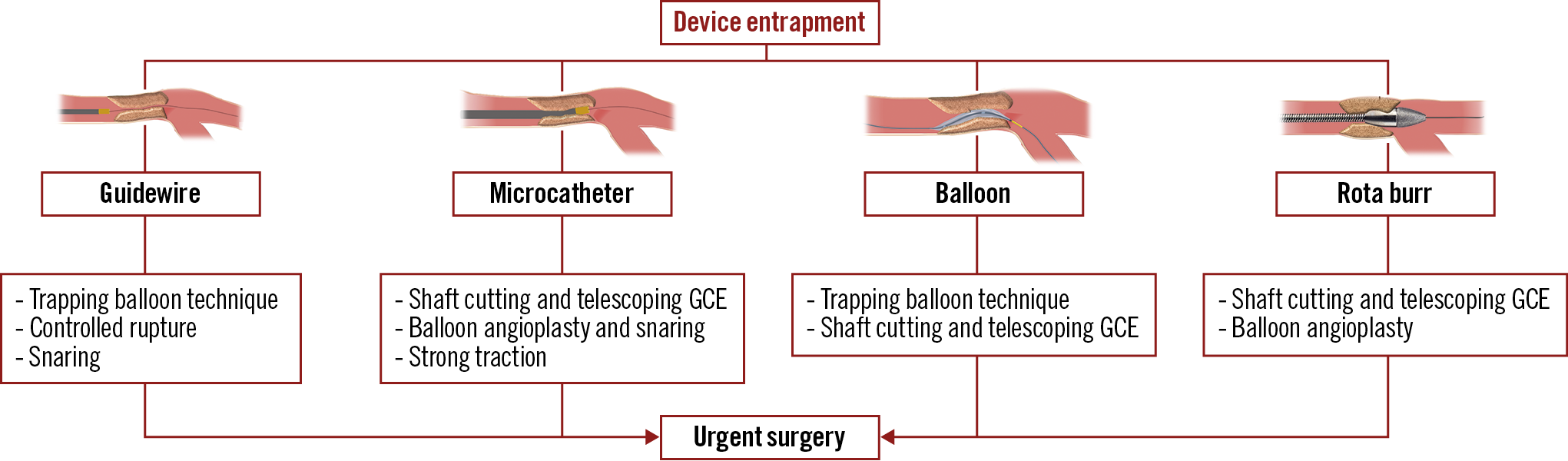
Figure 1. Techniques used for the management of device entrapment. GCE: guide catheter extension
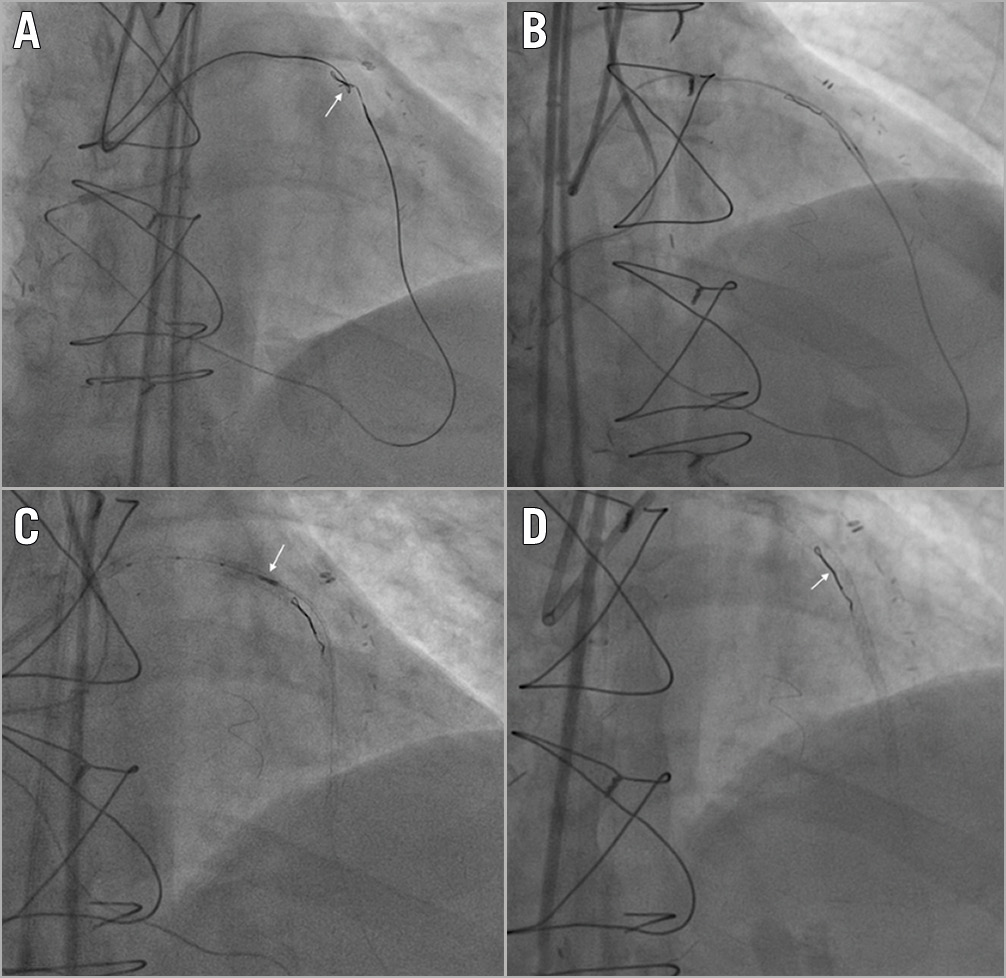
Figure 2. Guidewire entrapment: clean controlled tip rupture. A) A retrograde Gaia Second (Asahi Intecc, Aichi, Japan) knuckled wire knot (white arrow). B) Controlled rupture by simultaneous microcatheter pushing and guidewire pulling. C) IVUS evaluation (white arrow) for guidewire unravelling. D) Complete stent coverage of the unravelled guidewire fragment (white arrow).
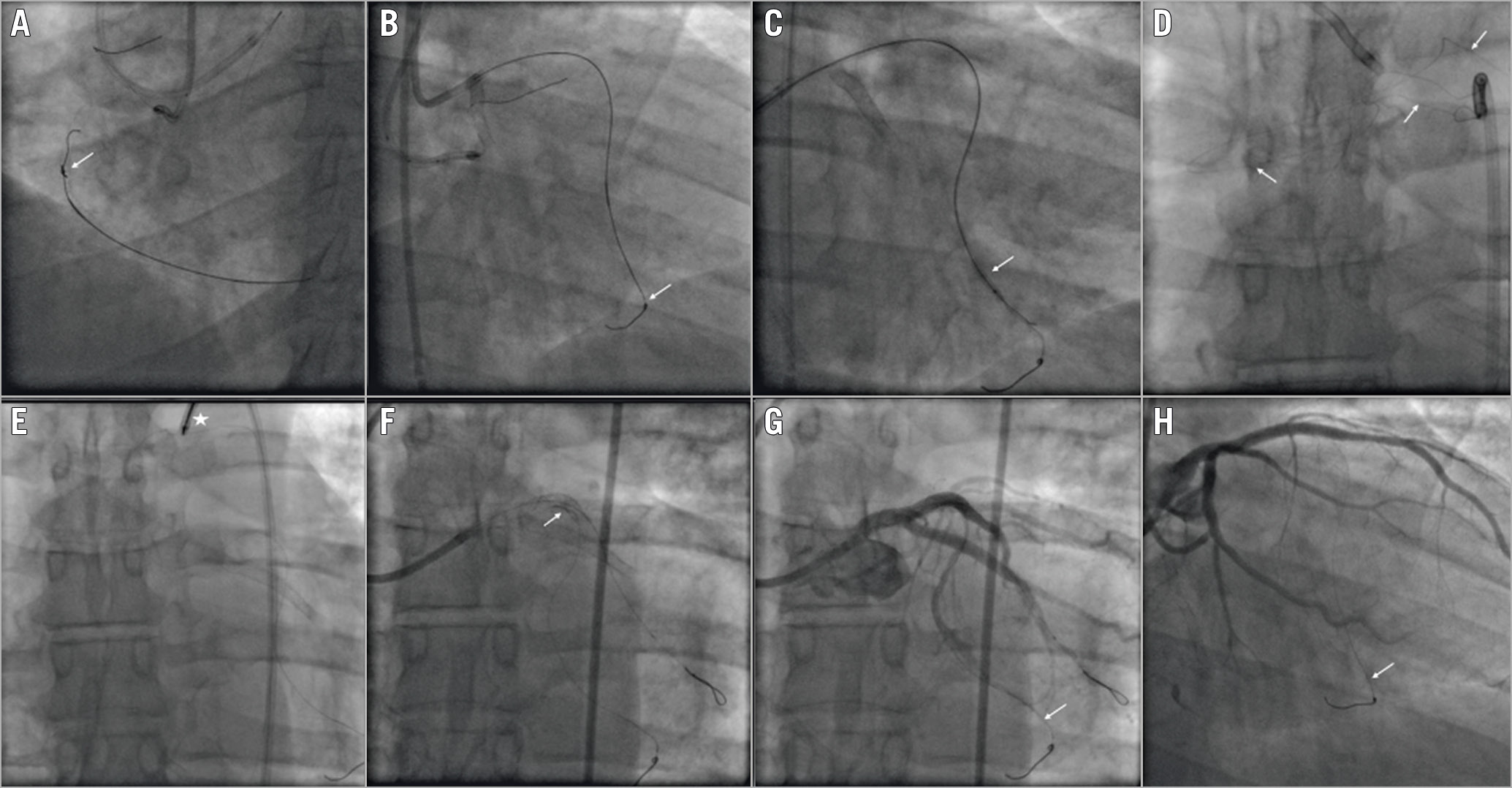
Figure 3. Guidewire entrapment: unfolding of the coil into small filaments. A) A retrograde ULTIMATEbros 3 (Asahi Intecc) guidewire knot (white arrow). B) Guidewire entrapment into the septal branch (white arrow). C) Balloon angioplasty around the entrapped guidewire (white arrow). D) Guidewire unfolding into a small filament (white arrows). E) Snaring of the unravelled guidewire fragment (white asterisk). F) Left main and proximal LAD stenting to trap the unravelled fragment (white arrow). G) Final result (white arrow showing the entrapped guidewire). H) Six-month coronary angiography follow-up (white arrow showing the entrapped guidewire).
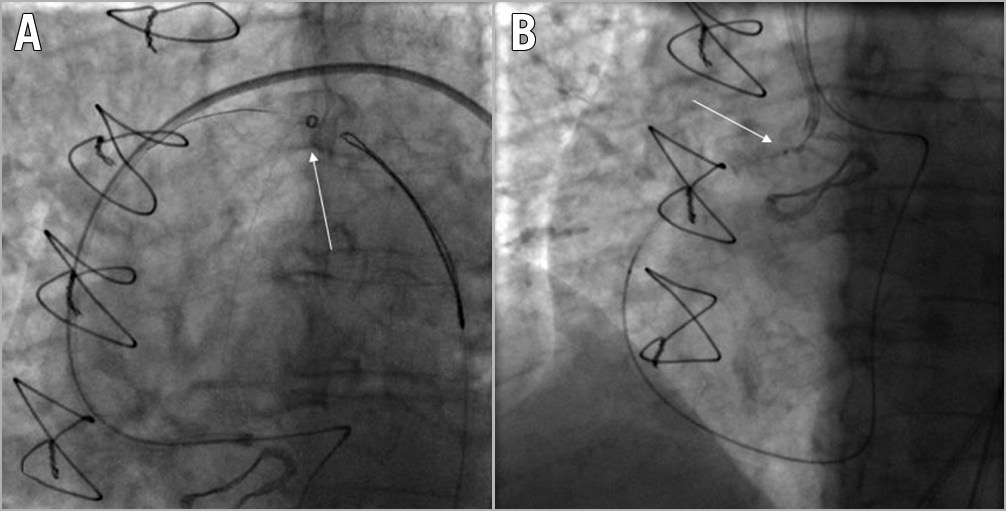
Figure 4. Guidewire entrapment requiring surgical removal. A) Snaring of an RG3 guidewire (Asahi Intecc) during a retrograde PCI of an ostial RCA CTO (white arrow). B) RG3 wire entanglement (white arrow) within the biological aortic prosthesis valve mesh.
Among the 6 cases of microcatheter entrapment, 5 took place during a retrograde CTO PCI. In 4 cases the entrapment occurred in small sharply angulated collateral branches. Successful retrieval was achieved by cutting the microcatheter shaft and advancing a guide catheter extension over the shaft, followed by gentle pulling of the entrapped microcatheter. In 1 case, a tip fracture of the microcatheter occurred within a very calcified CTO lesion during an ipsilateral (septal-septal) retrograde approach. The guidewire got stuck within the fractured microcatheter tip, allowing a successful retrieval of the fragment by pulling the retrograde wire (Figure 5). The only case of microcatheter entrapment that occurred during an antegrade approach was characterised by microcatheter tip fracture within the CTO body. A balloon angioplasty around the entrapped fragment managed to free the tip, allowing its successful retrieval with a snare.
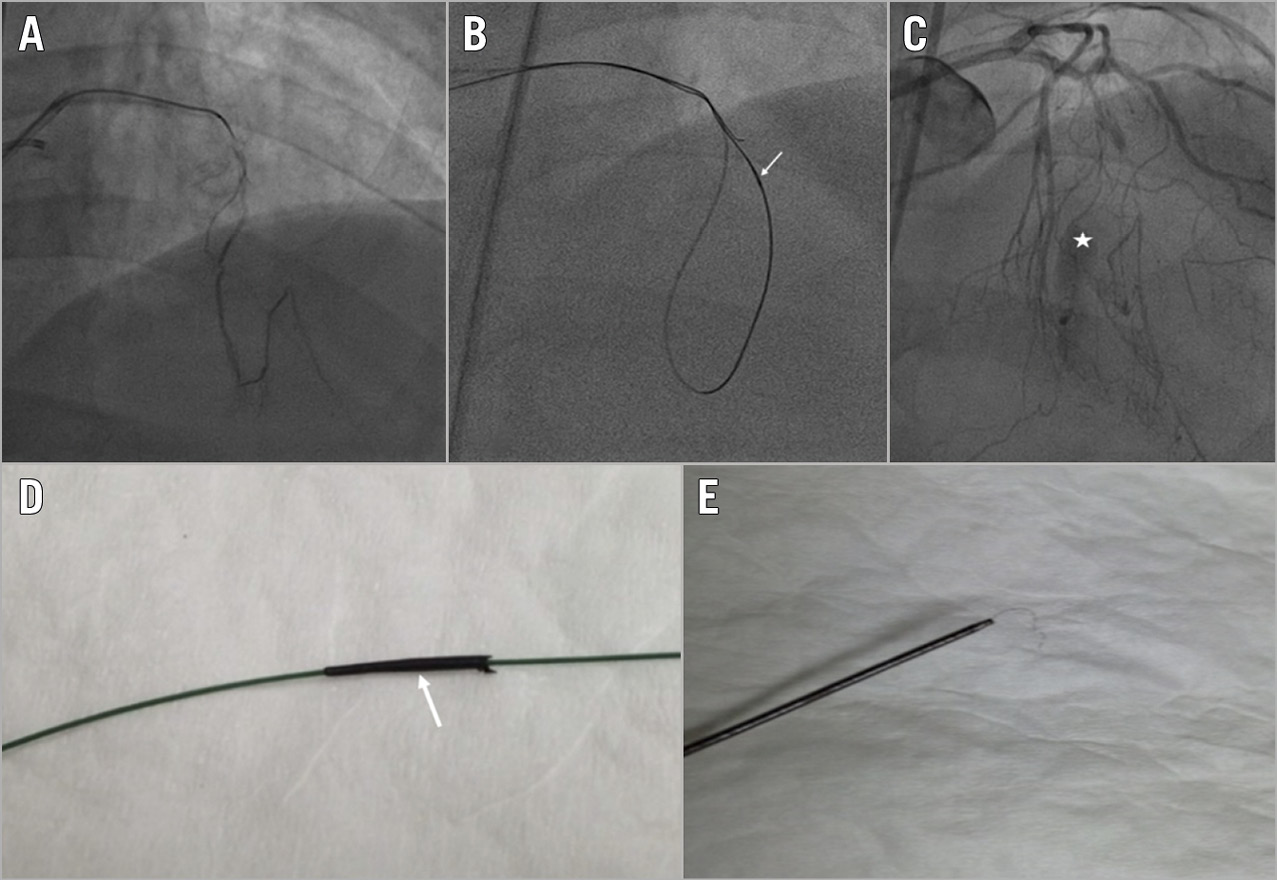
Figure 5. Microcatheter tip fracture. A) Selective injection of a septal-septal connection. B) Retrograde microcatheter (Corsair; Asahi Intecc) stuck in the CTO body (white arrow). C) Septal branch rupture after microcatheter retrieval (white asterisk). D) & E) Microcatheter tip fracture (white arrow).
Among the 6 cases of antegrade balloon entrapment, 4 were successfully retrieved with the trapping balloon technique (Figure 6), and 2 cases were solved by cutting the shaft, advancing a guide catheter extension and pulling the entrapped balloon. A single case of guide catheter extension entrapment occurred due to a shaft fracture. In this patient, a 2.0 mm balloon was advanced and inflated into the distal cylinder to trap the device fragment, then the gear was successfully retracted as a single unit (Figure 7).
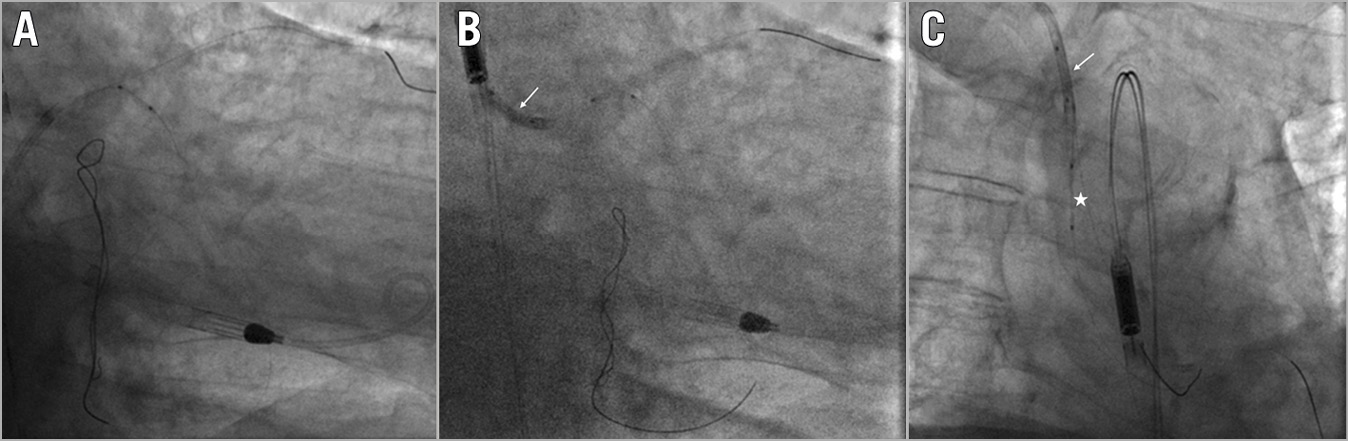
Figure 6. Balloon entrapment. A) Balloon-catheter shaft rupture. B) Trapping of the retained fragment with a balloon into the guide catheter (white arrow). C) Successful retrieval of the entrapped balloon (white asterisk) as a unit (white arrow indicating the trapping balloon).
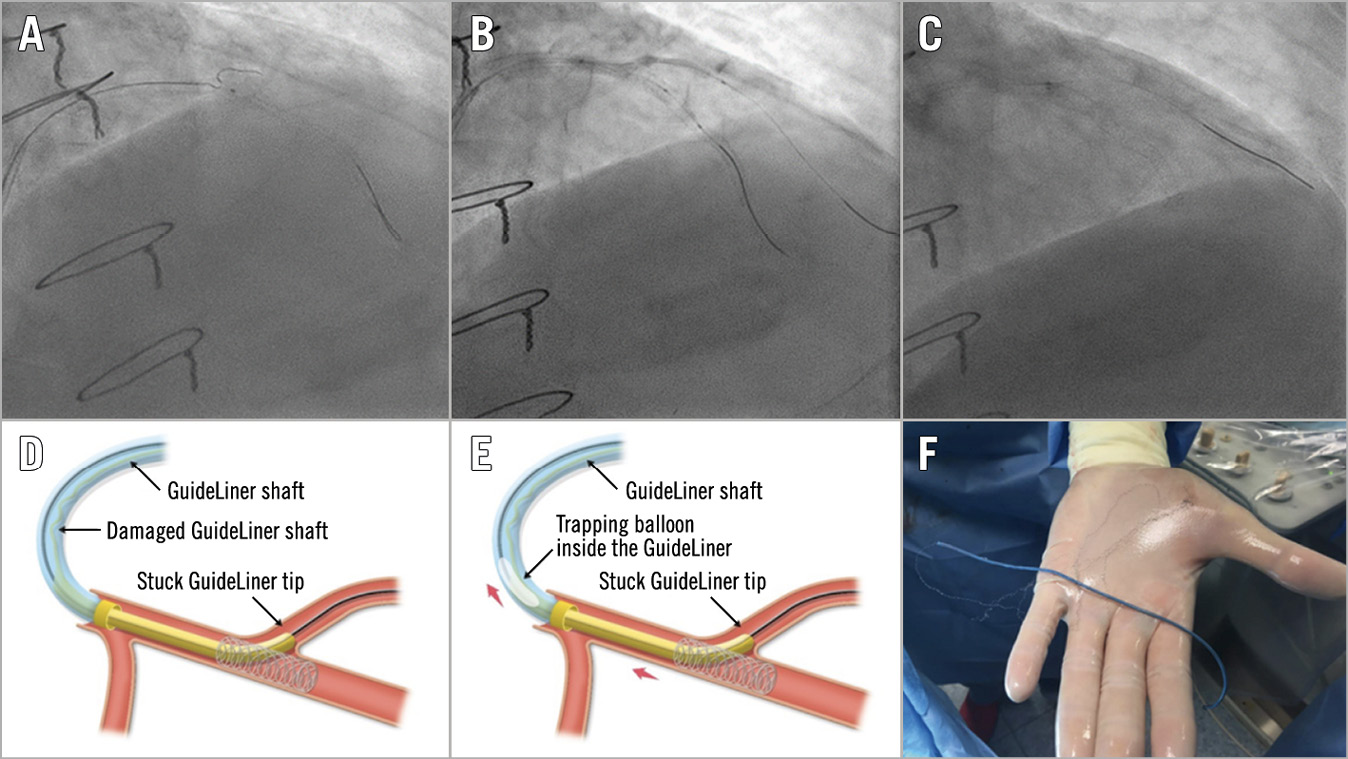
Figure 7. Guide catheter extension entrapment. A) Wiring of the diagonal branch. B) Balloon dilatation of the stent struts across the diagonal branch. C) Entrapment of the guiding catheter extension within the stent struts. D) Torn and damaged shaft of the guiding extension. E) Successful retrieval by inflating a balloon into the distal cylinder of the extension device followed by pulling the whole system out as a unit. F) Complete retrieval of the entrapped device.
Finally, 10 cases of rotational atherectomy burr entrapment were identified: in 8 cases the burr was removed cutting the Rotablator drive shaft sheath and advancing a guide catheter extension as close as possible to the entrapped burr, followed by withdrawal of the entrapped burr (Figure 8). In the remaining 2 cases, a balloon angioplasty around the entrapped burr managed to free the device, achieving a successful retrieval of the burr.
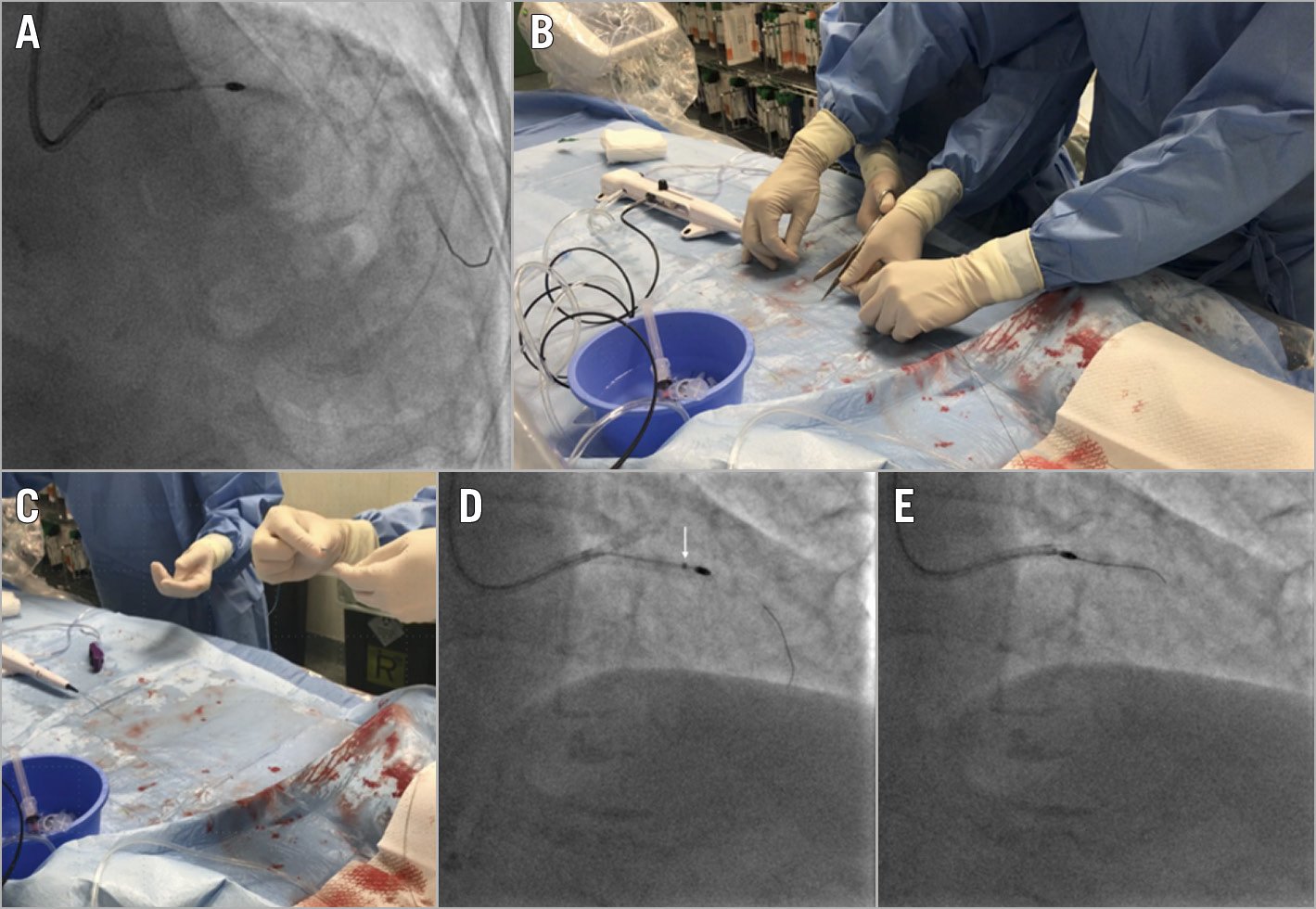
Figure 8. Rotational atherectomy burr entrapment. A) A 1.25 mm burr entrapped in the LAD. B) The Rotablator is disassembled by cutting the drive shaft sheath and the catheter shaft. C) Insertion of a guide catheter extension through the distal end of the system. D) Advancement of the guide catheter extension as close as possible to the entrapped burr (white arrow). E) Successful burr retrieval.
PROCEDURAL OUTCOMES
Overall technical success was achieved in 86.1% and procedural success in 80.5% of patients. During antegrade CTO PCI, technical and procedural success was achieved in 94.7% and 89.4% of the cases, respectively. However, during retrograde CTO PCI technical success was achieved in 76.4% of the patients and procedural success in 70.5%. Three patients (8.3%) developed an intraprocedural MI as a consequence of the retrieval manoeuvres: one of these also required urgent pericardiocentesis due to an Ellis type II perforation with impending tamponade. No periprocedural death or stroke was observed. Mean fluoroscopy time and contrast volume were 179±67 minutes and 249±126 mL, respectively (Table 2). Procedural and one-month outcomes are detailed in Table 4. At one-month follow-up, TLR was required in one patient (2.8%) with previous guidewire entrapment and stenting; one cardiac death (2.8%) occurred within 30 days, due to stent thrombosis of a non-target vessel.
Discussion
Our study describes the incidence and outcome of device entrapment in a large consecutive series of CTO PCI performed by high-volume experienced operators from five different centres in Italy. The main findings can be summarised as follows: 1) device entrapment is an infrequent complication (1.5%) during CTO PCI and most commonly consists of entrapped guidewires; 2) the incidence of device entrapment does not significatively differ between antegrade and retrograde CTO PCI; 3) device entrapment may be successfully managed percutaneously in most situations, allowing safe completion of the procedure; 4) overall periprocedural and one-month event rates are low in experienced hands.
Device entrapment during PCI is an infrequent but potentially life-threatening complication predisposing patients to an increased risk of vessel perforation, vessel thrombosis, and early and late myocardial infarction8,9,10. CTO PCI might increase the risk of device entrapment due to the presence of severely calcified and tortuous occluded vessels11,12. In addition, the aggressive wiring and ballooning techniques used during CTO interventions, as well as the retrograde advancement of equipment through small sharply angulated collateral branches, might increase the risk of device entrapment13,14.
As shown by our series, device entrapment does not only occur during retrograde CTO PCI. The incidence of device entrapment did not differ significatively between an antegrade and a retrograde approach (1.3% vs 1.9%, respectively, p=0.203). Notwithstanding, retrograde entrapped equipment was more hazardous and technically challenging to treat as compared with antegrade entrapped equipment and was associated with lower technical and procedural success rates.
Because it is a rare occurrence, most operators might not have enough experience concerning how to approach device entrapment. Furthermore, dedicated devices for retrieval manoeuvres may not be available in most catheterisation laboratories, or operators may not be familiar with their use.
The occurrence of device entrapment can be reduced by avoiding rapid advancement, excessive force or torquing to coronary devices, especially in small, tortuous and calcified lesions. Once entrapment has occurred, smooth traction should be applied as a first-line strategy in order to free the device. Post-entrapment modification of the plaque might be helpful, whenever possible15. Likewise, careful tension on the device should be applied by using microcatheters, snares, or telescopic guide extension catheters in order to provide a more focused and coaxial application of the withdrawal force, facilitating the retrieval of the device16,17,18,19.
However, device entrapment remains a feared complication, even by experienced CTO PCI operators, requiring an advanced knowledge of tools and dedicated techniques to retrieve the entrapped equipment successfully.
Limitations
There are several important limitations of our study. First, this is a retrospective study and it is thus subject to bias and confounding. Second, the lack of individual data from the entire cohort prevents us from comparing rates of procedural and follow-up adverse events between patients with device entrapment and those without, as well as identifying predictors of device entrapment. Third, the number of patients is limited, although comparable to many previously published CTO registry studies. Finally, all CTO PCIs were performed by highly skilled CTO dedicated operators and the results may not be generalisable to the daily clinical practice of less experienced operators.
Conclusions
This first report of a series of device entrapments in patients undergoing CTO PCI shows a low incidence and high procedural retrieval success rates by expert operators. Additionally, a low incidence of clinical events at one-month follow up was observed. An in-depth knowledge of potential complications and a structured approach to their management is essential for the interventional cardiologist to ensure prevention and optimise outcomes when complications occur.
|
Impact on daily practice This is the first study reporting the incidence and the management strategies of device entrapment during CTO revascularisation. A structured approach is an essential tool for the interventional cardiologist to optimise outcomes when these rare complications occur. |
Acknowledgements
J. Sanz Sanchez is personally supported by a grant from the Fundación Alfonso Martin Escudero (Madrid, Spain).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

