CASE SUMMARY
BACKGROUND: A 52-year-old female presented with acute anterior ST-elevation myocardial infarction (STEMI) within one hour of symptom onset to the emergency department. She was referred for urgent primary angioplasty.
INVESTIGATION: Physical examination, laboratory investigations, ECG, urgent percutaneous coronary intervention (PCI).
DIAGNOSIS: Single-vessel coronary artery disease (SVD).
TREATMENT: Intended to stent culprit lesion. However, stent dislodged in left main coronary artery (LMCA) during attempted PCI to diffuse mid segment of left anterior descending (LAD). Initial attempt failed to retrieve the dislodged stent with snare. Dislodged stent removed with multiple wire technique, complicated by severe dissection in LAD and left circumflex artery back into the LMCA. The stent was trapped at tip of 6 Fr right femoral sheath, unable to be withdrawn. What next?
KEYWORDS: Acute myocardial infarction, cardiogenic shock, circumflex dissection, emergency coronary artery bypass graft (CABG), intra-aortic balloon pump, left main dissection, percutaneous intervention, stent dislodgement.
PRESENTATION OF THE CASE
A previously well 52-year-old female presented with a first episode of acute onset chest pain. Her risk factors for coronary disease included hypertension, dyslipidaemia and a positive family history. The presenting electrocardiogram (ECG) showed anterior ST elevation. She was haemodynamically stable. Blood investigations showed a normal renal function and an unremarkable full blood count. She was loaded orally with aspirin (300 mg), clopidogrel (600 mg) and transferred for urgent primary angioplasty.
Coronary angiography was performed via the right femoral artery using a 6 Fr sheath. The diagnostic images revealed a short LMCA with luminal irregularities; the proximal LAD had an acute take-off from the LMCA and diffuse stenosis from the proximal to the mid segments. The mid LAD segment had two tandem lesions, proximally a 95% tubular culprit lesion and distally a calcified 70% diffuse lesion. The circumflex artery (LCX) was of large calibre with luminal irregularities. The right coronary artery (RCA) was dominant with mild to moderate calcification.
The culprit lesion was identified as mid LAD (Figure 1).
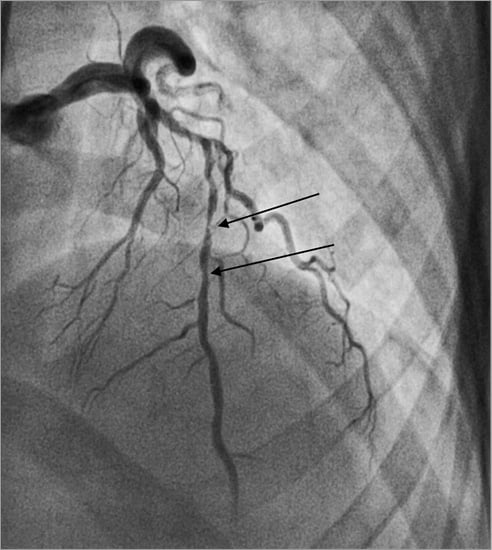
Figure 1. Culprit vessel, diffuse mid LAD disease.
Access for the intervention was via the right femoral artery using a 6 Fr guide catheter (EBU 3.5 Launcher; Medtronic, Minneapolis, MN, USA). Intracoronary heparin (7,000 units) was administered. The LAD was wired with a Fielder FC wire (ASAHI Intecc, Aichi, Japan). The culprit lesion in the mid LAD was predilated with a 2.0×8 mm MINI-TREK™ balloon (Abbott Vascular, Diegem, Belgium) to a maximum pressure of 18 atmospheres (Figure 2A and Figure 2B). As the patient was a non-diabetic and had SVD, the strategy for coronary intervention was to stent the mid LAD lesions. A 2.5×36 mm drug-eluting stent (BioMatrix; Biosensors, Morges, Switzerland) was not able to cross the mid LAD lesion. The decision was made to predilate the LAD lesions further. However, upon attempted withdrawal of the stent it became dislodged in the LMCA (Figure 3A and Figure 3B). An unsuccessful attempt was made to snare the stent from the LMCA using an Amplatz GooseNeck™ device (2.5; ev3 Inc., Plymouth, MN, USA). Two additional wires, a Fielder FC wire and a Runthrough wire (Terumo Corporation, Tokyo, Japan) were inserted and a knot was made in the distal LAD with counter-clocking of the distal wires upon each other. Forceful pullback of the knotted wires enabled removal of the stent from the LMCA. However, the dislodged stent was too large to pass through a right groin 6 Fr sheath (Figure 4). The left groin was punctured with an 8 Fr sheath to retrieve the dislodged stent from the contralateral groin. However, repeat diagnostic coronary angiography demonstrated coronary vessel dissection in the proximal LAD extending back into the LMCA and into the proximal LCX (Figure 5A and Figure 5B). The patient did not have chest pain and the BP remained stable. TIMI 2 flow was noted in the LAD and LCX arteries. A 7 Fr Judkins Left (JL) 3.5 (VISTA BRITE TIP®; Cordis, Johnson & Johnson, Warren, NJ, USA) guide catheter engaged the LMCA from the left groin. The left circumflex was cannulated and protected with a Runthrough wire with return of TIMI 3 flow. We were unable to wire across the grade 4 dissection flap in the proximal LAD. Further angiography showed acute occlusion of the proximal LAD. The patient became symptomatic with chest pain, further ST-segment elevation on ECG and rapidly developed cardiogenic shock. We now had the dilemma of vascular access as both groins were used: the right groin with 6 Fr sheath blocked with the dislodged stent, and the left groin with an 8 Fr sheath with a 7 Fr JL 3.5 guide supporting a PTCA wire cannulating the proximally dissected LCX, providing the sole left-sided circulation. What would you do next?
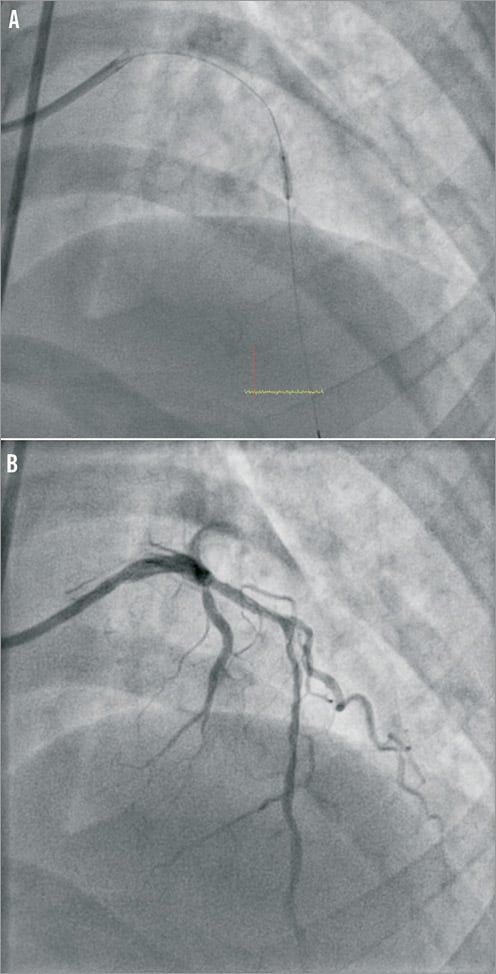
Figure 2. A) Predilatation to proximal and mid LAD with short 2.0 mm balloon. B) LAD post POBA with 2.0 mm balloon.
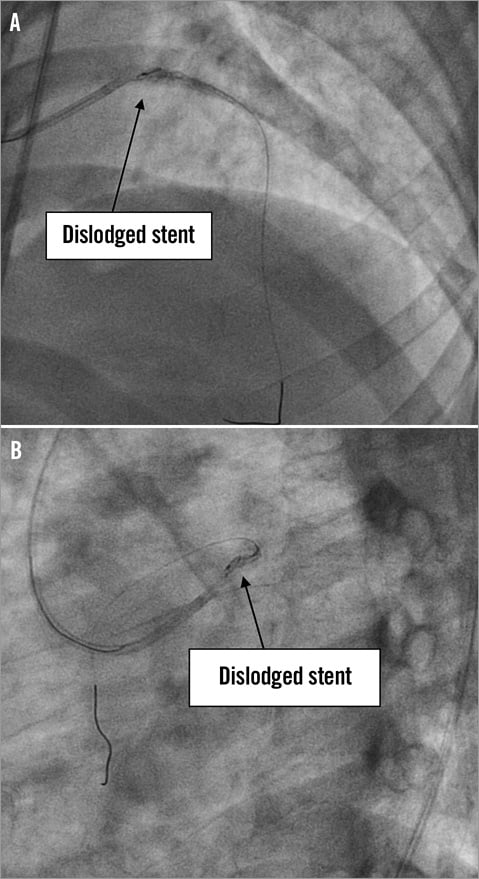
Figure 3. A & B) Stent unable to cross proximal LAD lesion. On pullback prior to further predilatation of proximal LAD, stent is dislodged at left main.
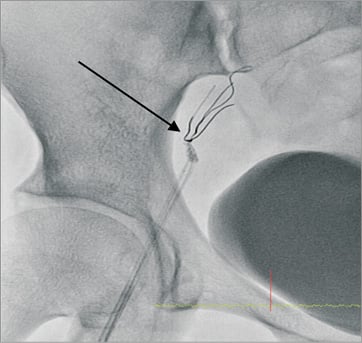
Figure 4. Collapsed stent caught at the tip of 6 Fr sheath.
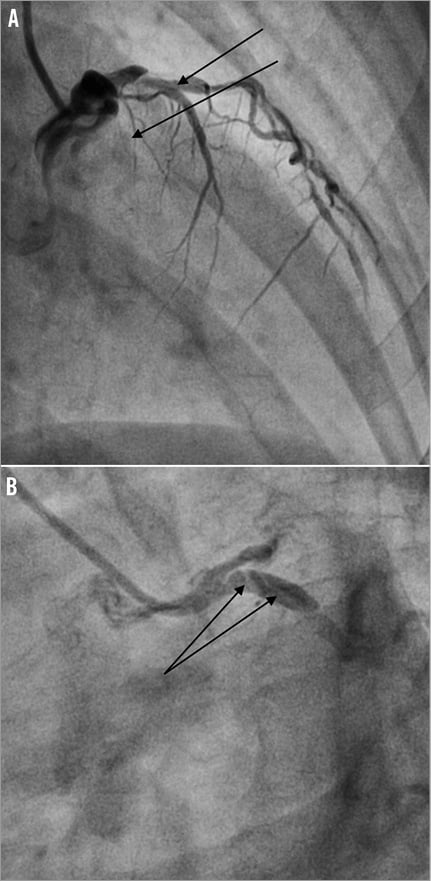
Figure 5. A) Occlusive dissection in proximal LAD extending back to LMCA and LCX. B) Proximal circumflex dissection in LCX.
How would I treat?
THE INVITED EXPERTS’ OPINION
This is a very tough situation.
Anterior STEMI and now LM/LAD and LCX dissection with shock. Without revascularisation she has a very high mortality risk. A salvage surgical opinion is needed consistent with some potential better outcome as reported in the initial SHOCK trial.
The operators are not keen to remove the circumflex wire since, if this occludes, an even worse situation may arise. Try radial access with a second guide to rewire the circumflex. You can then pull the femoral sheath and system and employ mechanical support (as she is in shock, she would now probably need tandem heart, ECMO or some other circulatory support rather than just an intra-aortic balloon pump).
Two options:
1. Less favourable option in a 52-year-old: if haemodynamics improve significantly, you could stent left main into circumflex and accept a large anterior myocardial infarction.
2. More favourable option in a 52-year-old: if haemodynamics improve significantly, you could stent left main into circumflex and then transfer to surgery for immediate grafting to left anterior descending to limit the size of anterior infarct. For speed and immediate high coronary flow use a saphenous vein graft. If possible, consider off-pump (if haemodynamics permit), otherwise on-pump beating heart (i.e., no cardioplegic arrest). Also consider grafting the circumflex. Use circulatory support postoperatively with daily transoesophageal echo after three to four days to assess cardiac function and guide removal of circulatory support.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
For patients with acute coronary syndrome, the culprit lesion needs to be treated first and the operator has to assess the risks of percutaneous coronary intervention (PCI) according to the lesion, the artery and the patient. In this particular case, the discussion should be focused on three main points.
First, failure to cross the lesion with a long stent
The Extra Backup guiding catheter usually gives enough support. In case of tortuosity and calcifications, the guidewire should have intermediate or extra support. After predilatation, if the stent cannot cross the lesion, another wire can be placed in the left circumflex artery (LCX) as a buddy wire to increase the support or in a proximal septal branch for anchoring the balloon1. In case of failure, rotational atherectomy can be an option to treat this lesion and to facilitate stent delivery by debulking proximal calcifications. If the stent cannot progress in proximal tortuosity with calcifications, a monorail or a “mother-and-child” guide catheter extension2,3 can be placed in the proximal left anterior descending coronary artery (LAD). In this case, a deep intubation of the guiding catheter (GC) increases active support but could induce proximal dissection.
Second, stent dislodgement in left main coronary artery (LMCA)
The stent withdrawal should always be carried out with caution. By spider or cranial views, we should check the alignment of the guiding catheter and the wire before stent retrieval. An important angulation between GC and guidewire leads to stent dislodgment. Repeated dye injections, the use of two other knotted wires and three wires with the dislodged stent increase the risk of extensive dissection or thrombosis. Moreover, the culprit lesion is in the LAD and we should not treat another artery or lesion in such a situation without cardiogenic shock4. By pushing it in the LAD with a balloon, it is maybe safer to deploy or to crush the dislodged stent in the proximal target vessel.
Third, how can we manage a patient in cardiogenic shock with occlusive iatrogenic dissection in proximal LAD extending to LCX, LMCA and sometimes in ascending aorta5?
In case of distal extensive dissection, it can be very difficult to graft the distal true lumen and an emergency coronary artery bypass graft (CABG) is probably not the best option. Haemodynamic support such as an intra-aortic balloon pump (IABP) via the left groin can be discussed, before using a 7 Fr GC (or 7.5 Fr sheathless GC) by radial approach. Other angiographic views, without repeated dye injections, could help to place wires in the distal true lumen. In bifurcation lesion with occlusive dissection in both branches, a strategy with two stents could be safer to achieve complete revascularisation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The patient was reviewed by the on-call cardiothoracic surgical consultant. Emergency CABG was agreed to be the best management strategy with the insertion of an intra-aortic balloon pump (IABP; Datascope, Fairfield, NJ, USA). Prior to transfer to the operating room a short bare metal stent, Vision 3.5×12 mm (Abbott Vascular, Diegem, Belgium), was deployed to cover the proximal left circumflex dissection flap restoring TIMI 3 flow (Figure 6). An IABP was then inserted from the left groin and commenced on inotropes. The patient was transferred to the operating room for urgent single-vessel CABG (saphenous vein graft to LAD) and surgical removal of the dislodged coronary stent from the right groin. The stent was then sent to the histopathology laboratory (Figure 7). Histology of the tissue removed from the dislodged stent demonstrated stripped coronary intima and media with possible DES polymer (Figure 8). The patient made a rapid recovery from the emergency CABG. Post CABG a resting transthoracic echocardiogram showed preserved left ventricular ejection fraction of 52% and no Q-waves on her ECG. She was discharged home seven days later. On subsequent review seven months later she remains asymptomatic with a negative stress myocardial perfusion scan for ischaemia.
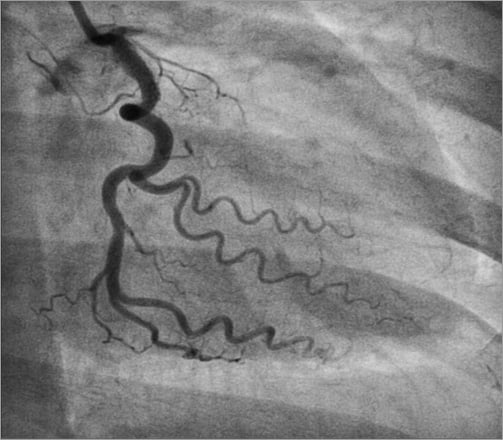
Figure 6. Short 3.5×12 mm BMS covers proximal circumflex dissection and restores TIMI 3 flow. The proximal LAD is still occluded. The patient is transferred for emergency CABG (SVG to LAD).
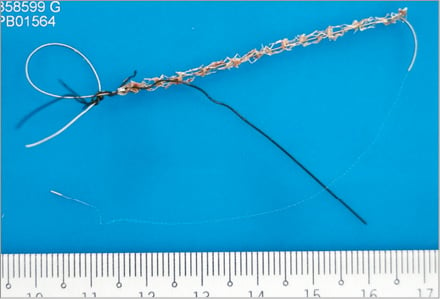
Figure 7. Tissue trapped within the wire meshwork of the stent was retrieved for histological examination.
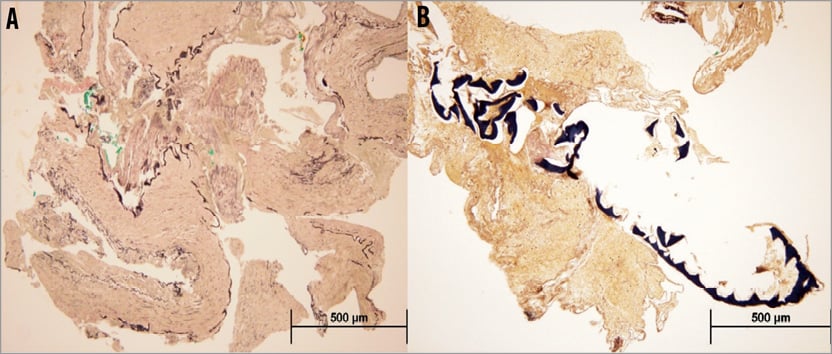
Figure 8. Elastica van Gieson staining technique demonstrated the presence of the internal elastic lamina in the arterial segments (A), as well as a foreign body entrapped within the fibrin clots (B). The overall morphological features were in keeping with a muscular artery such as a coronary artery.
Discussion and conclusion
This case highlights the well documented complication of coronary stent dislodgement. Potentially lethal associations can ensue: stent embolisation, thrombosis and vessel dissection resulting in myocardial infarction and the need for emergency CABG6. However, with improved stent technology and now with premounted stents, the incidence of stent dislodgement has fallen from the 8% originally reported (when stents were manually crimped) to less than 0.5% implanted over a five-year period7,8. In calcified angulated coronaries, a deep-seated wire with coaxial guide position and lesion modification with predilatation or rotablation reduces the risk of stent displacement from balloon. For this case, further predilation might have decreased the risk for stent dislodgment. The withdrawal of the stent from a non-coaxial guide angulation further contributed to the stent dislodgement.
Several options regarding the management of a dislodged stent have been reported. The displaced stent can be crushed with a second stent9, be deployed with rewiring (buddy wire technique)10 with a smaller balloon, may be retrieved (various methods), or result in emergency CABG. Depending on the clinical and interventional scenario, a specific technique may be pursued. In this case, we opted initially for an attempt at stent retrieval. Various strategies have been reported. We used the braiding technique, with several wires intertwined in a counter-clockwise twist to form a knot distal to the stent. This allows retrieval of the dislodged stent when the wires are removed11. Other methods described are the use of a retrieval device, commonly a snare or rarely a bioptome device12. Another technique described includes passing a smaller balloon distal to the stent to help withdrawal of the displaced stent13. In the majority of cases a retrieval method is successful. However, as in this case, there is a high risk of injury to the native vessel.
For complications involving the LM, the need for surgical revascularisation should be discussed promptly with the cardiothoracic team. In this case, the surgeons chose to do a single bypass to the LAD with a saphenous vein graft in order to perfuse her LAD as soon as possible, as this incident occurred in the setting of a primary PCI for acute anterior STEMI. The CABG was performed on cardiopulmonary bypass with the heart beating as only the LAD territory was ischaemic after the left circumflex artery was stented. The anastomosis was done with an intracoronary shunt and Medtronic Octopus® Evolution coronary stabiliser.
Conflict of interest statement
The authors have no conflicts of interest to declare.

