Case summary
Background: A 53-year-old female was sent for diagnostic angiography after successful reperfusion therapy for an anterior ST-elevation myocardial infarct. The culprit lesion was a LAD/D1 bifurcation stenosis.
Investigations: Coronary angiography, intravascular ultrasound.
Diagnosis: Left anterior descending artery/first diagonal artery bifurcation stenosis, fractured jailed side branch wire.
Treatment: Provisional stenting strategy for bifurcation stenosis. Consideration of surgical and percutaneous options to retrieve fractured, jailed, side branch wire. Wire and balloon catheter wrap technique for retrieval of fractured wire.
KEYWORDS: Provisional stenting, bifurcation stenosis, STEMI, fractured wire
Presentation of the case
A 53-year-old non-diabetic female presented with an acute anterior ST-segment elevation myocardial infarction. She was treated with fibrinolytic therapy with resultant >70% ST-segment resolution. Peak cardiac troponin-T was elevated at 2.82 ng/dL. Routine, early coronary angiography was undertaken via the right radial artery. This revealed a right dominant circulation with single vessel disease. The culprit lesion was a bifurcation stenosis in the mid left anterior descending artery (LAD) at the level of the first septal perforator and a large first diagonal artery (D1) (Medina class 1, 1, 1). The proximal segment of the D1 had moderate smooth stenosis of 50% severity (Figure 1).
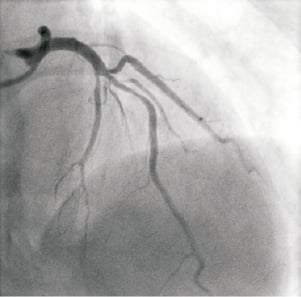
Figure 1. Culprit bifurcation lesion in mid LAD at S1/D1 trifurcation (RAO 5° cranial 40°)
The approach for the bifurcation stenosis was to provisionally stent the LAD using a strategy of wiring both LAD and D1 with direct stenting of the mid LAD without pre-dilation of the D1 side branch. A 6 Fr Kimny® guiding catheter (Boston Scientific Corp., Natick, MA, USA) was used. Intra-arterial heparin was given to achieve an activated clotting time of more than 250 seconds. The LAD was wired with a Balance Middle Weight (BMW) universal 0.014 inch guidewire (Guidant Corp., Santa Clara, CA, USA) and the diagonal side branch with the same guidewire. The LAD was directly stented with a 3.5×20 TAXUS® Liberté™ stent (Boston Scientific Corp., Natick, MA, USA), deployed at 15 atmospheres, thus jailing the diagonal side branch wire. Angiographically the stent appeared under deployed (Figure 2), so was post-dilated with a 4.0×12 Quantum™ Maverick® balloon catheter (Boston Scientific Corp., Natick, MA, USA) to 20 atmospheres. At this stage the D1 was rewired with a PILOT 50 0.014 inch wire (Guidant Corp/Abbott Vascular, Redwood City, CA, USA) to facilitate balloon angioplasty of the D1 which had the angiographic appearances of plaque shift without compromise of flow. Whilst attempting to remove the trapped BMW wire, it fractured leaving the distal radio-opaque portion in the D1, trapped under the stent with an unraveled filament extending back into the guide catheter (Figure 3).
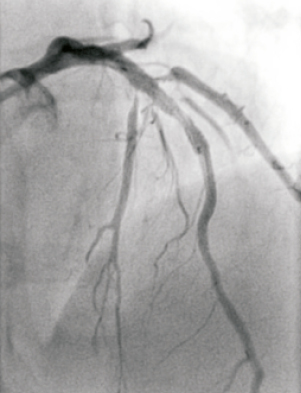
Figure 2. Angiographic result after initial stent implantation. Note the underdeployed stent and plaque shift into the first and second diagonal.
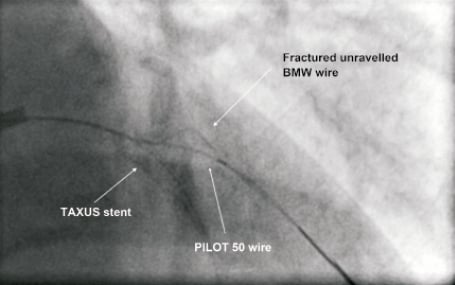
Figure 3. Fractured unraveled jailed BMW wire in D1.
How could I treat?
The Invited Experts’ opinion
Figure 4 shows the OCT appearance of a jailed side branch (SB) wire after main vessel (MV) stenting. The possibility of having difficulties in removing it represents a major drawback of the “provisional” approach in the treatment of bifurcation lesions.

Figure 4. OCT appearance of a jailed wire.
According to our experience a series of simple rules (Table 1) prevents the occurrence of jailed guidewire entrapment. Indeed, the application of these rules is associated with no guidewire fracture in an open series of 1,291 consecutive bifurcated lesions treated according to a provisional approach at our institution. Interestingly, some of these rules have not been applied in the present case (it is therefore possible that there is a too distal placement of the jailed wire, possible stent oversizing for the distal MV or high-pressure balloon post-dilation with jailed wire in place).

In any case, once jailed wire entrapment has occurred such as we see here, a series of the following possible approaches can be used to solve the problem:
First of all, the coronary guidewire structure should be taken into account. Indeed, the coronary guidewires are basically constituted by an inner part (metal core) and by a metallic filament wrapped around the distal part of core and extending over it. Jailed guidewire entrapment under the stent struts is usually caused by the second part of the wire being stuck between the vessel wall and the MV stent struts. As a consequence, the application of increasing the pulling force at the shaft (core) of the wire may cause unravelling of the distal, external filament (the situation which probably occurred in the reported case). Thus, the chances to successfully remove the guidewire intact are increased, if the pulling force is applied as much as possible close to the retained tip. To do this, guiding catheter deep intubation can be used to reach the proximal part of the stent in order to transmit the pulling force as much as possible to the distal segment of the entrapped wire. Possible alternatives, applicable even after wire unravelling has occurred (like in the present case) are represented by the advancement of low profile rapid exchange balloons or low-profile over-the-wire balloons (after wire extension) over the entrapped wire to try and engage, and then enlarge the space between the MV vessel wall and the MV stent struts. Theoretically, penetrating microcatheters, like Tornus and Corsair (Asahi Intecc., Aichi, Japan) may also be considered for this purpose due to their higher “pushability” and due to the fact that they have been used to remove an entrapped wire in a calcific vessel1.
Finally, the worse situation faced is when the entrapped wire, after unravelling, is broken inside the coronary artery. Both surgical removal and conservative follow-up have been successfully used to manage patients with retained jailed wire fragments2. Among the interventional techniques, a commonly adopted strategy is to use snare systems. Yet, some other possible alternatives can be considered and are listed in Table 2.
Conflict of interest statement
The authors have no conflict of interest to declare.
How could I treat?
The Invited Expert’s opinion
Jailed wire fracture is rare, but a serious complication of percutaneous coronary bifurcation interventions.
Firstly, insertion of a wire into the side branch must be seriously considered because of the risks of the jailed wire technique. In this specific case, the ostium of the diagonal branch was stenotic; however, the lesion was not critical as seen in Figure 1. In my opinion, in this case it could be possible to intervene without using the jailed wire technique, even though the jailed wire technique is preferred by most operators.
Secondly, the side branch must be rewired by a second wire and the jailed wire removed after main branch stenting with low atmospheres, followed immediately by postdilatation with high atmospheres could be done. In this particular case a TAXUS Liberté stent (3.5×20) was deployed at 15 atmospheres and was post-dilated with a Quantum Maverick balloon (4.0×12) with 20 atmospheres which makes it particularly difficult to remove the jailed wire and may cause its fracture.
Ruptures of the jailed wires are described in literature1, however, to my knowledge, brief descriptions of successful interventional management are lacking.
In this case, the removal of the broken wire will be very difficult or even impossible, because a certain amount of force will be needed to pull the jailed and already broken wire, especially where high atmospheres were used.
I would advise to try using the technique of snaring with a loop-snare device the proximal end of the filament. Before pulling the system out, I would try to introduce another, probably harder, guiding wire under the stent struts into the diagonal branch and use a small balloon to dilate the space between vessel wall and struts. After that I would try to pull the system out. In the literature, successful retrieval of fragments of devices and catheters with the snaring device technique has been described, however, not for a jailed wire2.
In case this does not work, another suggestion would be to put a balloon (for example a 2.5×20 balloon) on another wire and inflate it with 20 atmospheres in the guiding catheter, and with the inflated balloon press part of the filament to it; then remove all the system together with the guiding catheter from the coronary artery. Hopefully the jailed wire will be retrieved; however, I do not personally believe that chances of success are very high.
If those interventional attempts are done without success, then surgical removal of the broken wire is needed3.
Conflict of interest statement
The author has no conflict of interest to declare.
How did I treat?
Actual treatment and management of the case
This complication is particularly challenging to deal with as a portion of the trapped wire is pinned underneath the deployed LAD stent. In order to manage this complication, we considered the following options:
Surgical option
(a) Contact the on call surgeon and request surgical retrieval.
Percutaneous options
(a) Inflate a balloon in the guide to trap the unravelled filament. By withdrawing the guide and the inflated balloon simultaneously, tension is maintained on the guidewire filament in continuity with the portion trapped behind the LAD stent and to the diagonal. The end result is to maintain sufficient constant traction on the wire to pull it free in its entirety1.
(b) Snare retrieval by capturing the radio-opaque segment in the D1. Alternatively, an attempt to snare the end of the unravelled filament in the guide could be attempted.
(c) Stent to exclude the fractured wire. This requires stenting from the left main artery into the LAD and most of the large D1. There will still be a fine filament free in the aorta after this approach.
(d) Deliberate “wire wrap” tangle to remove the trapped wire by using multiple angioplasty wires2.
Retrieval technique
The patient was haemodynamically stable without electrocardiographic or clinical evidence of ischaemia. An ACT was re-checked and the patient received a top-up dose of heparin. The following retrieval steps were performed.
1. An initial strategy of inflating a balloon in the guide proved unsuccessful at retrieving the fractured wire.
2. Snare retrieval of the wire was hampered by the small caliber of the D1 relative to the snare device and was ultimately unsuccessful despite several snares being used.
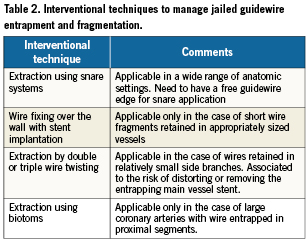
3. Wire wrap technique. This approach introduces multiple angioplasty wires to deliberately entangle the fractured wire in order to retrieve it. Multiple non-hydrophilic 0.014 inch angioplasty wires were placed in the D1 and LAD, rotated in a helical fashion to entangle the trapped wire (Figure 5). Once the wires were pulled back into the guide, the radio-opaque portion was mobilised into the aorta, though still trapped distally under the original stent (Figure 6).
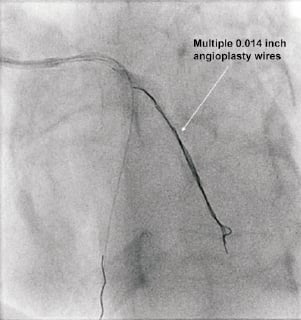
Figure 5. Wire wrap technique: Multiple non hydrophilic angioplasty wires in the diagonal. Deliberately rotate them in a helical fashion to entangle the trapped wire.
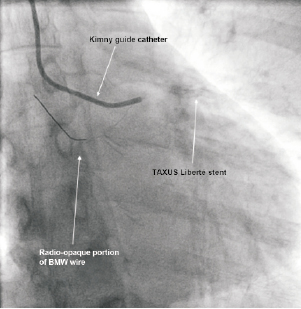
Figure 6. Radio-opaque portion mobilised to the proximal aorta but the wire is still trapped distally under and now also through the stent.
4. Removal of the radio-opaque segment from the aorta. To facilitate removal of the wire from the aorta, a second guide (7 Fr) was introduced via the right femoral artery. By using a goose-neck snare, the radio-opaque portion of the wire was partially retrieved (Figure 7). The radio-opaque portion broke off and a thin filament remained trapped under the stent and now also looped back through the stent extending into the aorta. This filament was very thin and firmly trapped by the stent. We could not successfully retrieve it using snare devices.
Figure 7. Radio-opaque portion in aorta gooseneck snared via a 2nd guide femorally. Wire partially retrieved but a thin filament broke off and remained trapped by the LAD by the stent.
5. Wire and balloon catheter wrap technique. Further attempts to remove the wire fragment in the LAD were unsuccessful using multiple wire tangles. Multiple wires and a single previously inflated balloon (deliberately inflated to increase its profile) were then placed in the LAD and diagonal, rotated in a helical fashion to deliberately trap the filament in the tangle. Upon traction, the trapped wire along with the LAD stent were entirely removed from the coronaries and retrieved from the guide (Figures 8-10).
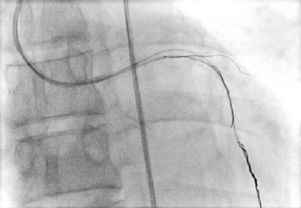
Figure 8. Multiple wires and a single balloon were then placed in the LAD and diagonal and rotated in a helical fashion to deliberately trap the filament in a tangle.

Figure 9. Picture of the retrieved fractured wire, stent and multiple wrapped wires with balloon.
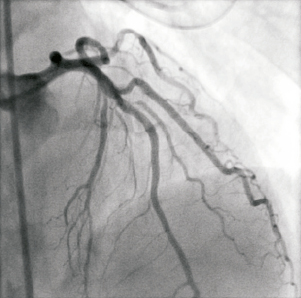
Figure 10. Post retrieval angiogram of LAD/D1 after successful removal of fractured wire and stent. Small dissection in diagonal evident though TIMI III flow.
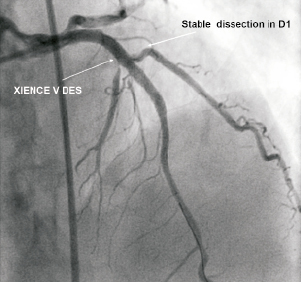
Figure 11. End result. A 3.5/28 XIENCE V® stent placed and optimised with a 4.5/8 Quantum balloon (IVUS guided). Stable dissection in D1 and D2 (small) now occluded. Undamaged left main artery after retrieval of stent and wire (IVUS)
6. Final result. A 3.5/28 XIENCE V® stent (Abbott Vascular, Santa Clara, CA, USA) was placed in the mid LAD as originally planned and optimised using IVUS and a 4.5/8 Quantum balloon. The end result is a shown in Figure 7, with a stable dissection in D1, an occluded small D2 and an undamaged left main artery after retrieval of stent and wire as assessed by IVUS (Figure 11).
The patient was discharged the next day without complications and is well at one year clinical follow-up.
Discussion
Percutaneous coronary intervention for coronary bifurcations remains technically challenging and accounts for up to 20% of coronary interventions3. The provisional strategy of implanting one stent in the main branch is considered by most as the default approach for the majority of bifurcations lesions and the technique of placing two angioplasty wires for bifurcations, one in the main branch, one in the side branch and “jailing” the side branch wire after stent implantation is well described3. Complications such as device dislodgement and wire fracture are uncommon with this approach but can occur in up to 0.02% of cases2.
The majority of complications that occur in interventional cardiology stem from our decision making process and from the underestimation of the downstream effect of those decisions4. In this case, one potential disadvantage of directly stenting the main branch without predilation is that it increased the chances of further post dilation, resulting in firmly jailing the wire between the side branch and the deployed stent. The net effect was to increase the chance of fracturing the wire upon attempted retrieval. However, when complications do occur, it is important to think calmly and to approach the problem in a rational and logical manner. In this case, the patient was stable, thus affording us the opportunity to ensure anticoagulation was appropriate, ask the opinions of our interventional and surgical colleagues and devise an appropriate strategy for wire removal. A fractured “jailed” side branch wire is an uncommon complication of a provisional bifurcation stenting strategy where the side branch is wired and subsequently jailed. If it occurs, the complete removal of the fractured side branch wire is very challenging as the jailed portion is behind a deployed stent. As in this case, retrieval is further hampered by the smaller calibre of the side branch. This case report highlights a variety of percutaneous retrieval techniques that include using multiple wires and a balloon catheter in a deliberate tangle to completely and effectively retrieve a jailed fractured angioplasty wire.
Conflict of interest statement
The authors have no conflict of interest to declare.