Abstract
Provisional stenting has become the default technique for the treatment of most coronary bifurcation lesions. However, the side branch (SB) can become compromised after main vessel (MV) stenting and restoring SB patency can be difficult in challenging anatomies. Angiographic and intracoronary imaging criteria can predict the risk of side branch closure and may encourage use of side branch protection strategies. These protective approaches provide strategies to avoid SB closure or overcome compromise following MV stenting, minimising periprocedural injury. In this article, we analyse the strategies of SB preservation discussed and developed during the most recent European Bifurcation Club (EBC) meetings.
Introduction
Provisional stenting has become the default technique for the treatment of most coronary bifurcation lesions. This strategy has also been called the simple approach; however, sometimes the procedure is not so simple – the side branch (SB) can occlude after main vessel (MV) stenting in up to 6-18% of cases12345. Restoring SB patency can be difficult, and the fear of having to deal with this situation may persuade operators to adopt an elective two-stent strategy despite anatomical suitability for stepwise provisional stenting. There are many ways to protect and restore side branch flow. In this article, we analyse the strategies of SB preservation during provisional stenting discussed in the last meeting of the European Bifurcation Club (EBC).
SB relevance
Consistent with the Bifurcation Academic Research Consortium (Bif-ARC) consensus6, an SB should be defined as “relevant” if the reference vessel diameter is ≥2.0 mm and represents a significant territory (>10%) of the myocardium, impacting prognosis. In the consensus6, an algorithm with a few scores to determine SB relevance has been proposed. The minimum requirement to assess the relevance of an SB is a baseline coronary angiogram in 2 orthogonal views, which allows indirect measurements (SB length, anatomical scores) that are surrogates of the SB-related myocardial mass. In this respect, an SB length ≥73 mm is assumed to supply ≥10% of myocardial mass7. However, in common practice, many operators consider a relevant SB a vessel ≥2.0 mm that they are unwilling to lose in the context of a specific patient anatomy. If the SB is considered relevant, then steps should be taken to protect it.
Predictors of SB occlusion
The mechanism of SB occlusion is a combination of carina shift and longitudinal plaque shift. However, the most prevalent cause of SB occlusion is over-dilatation of the distal part of the MV stent during the initial implantation. This is most prevalent in anatomies that are already predisposed to SB closure. To avoid this risk, it is crucial to implant the first stent according to the diameter of the distal MV and to finalise the implantation with the proximal optimisation technique (POT) above the carina level. The predisposing factors for SB occlusion have been identified in several studies. By intravascular ultrasound (IVUS), a carina with a spiky morphology (an “eyebrow” sign) has been found to be a powerful predictor of ostial SB damage after MV stent implantation8. By optimal coherence tomography (OCT), high lipid content of the MV lesion and a contralateral location of lipid in the bifurcation area can contribute to SB occlusion after provisional stenting9. A narrower carina tip angle, a shorter length between the proximal branching point to the carina tip and calcified plaque in the MV have also been proposed as OCT factors for SB complication1011. Other angiographic characteristics have been reported as predictors of SB occlusion, such as the diameter stenosis in both branches, lesion length, bifurcation angle or a thrombus-containing lesion12. A risk stratification score system (the RESOLVE [Risk prEdiction of Side branch OccLusion in coronary bifurcation interVEntion] score) was developed to help predict side branch occlusion4. This score includes 6 independent variables with different weights in the model: plaque distribution (on the opposing or same side of the SB), Thrombolysis in Myocardial Infarction (TIMI) flow grade of the MV before stenting, preprocedural diameter stenosis of the bifurcation core (%), bifurcation angle, diameter ratio between MV/SB and diameter stenosis of the SB before MV stenting (%). Patients with a score ≥10 points are considered a high-risk group, with a probability of SB occlusion close to 20%. A theoretical limitation of this score is the inclusion of 4 quantitative coronary angiography (QCA) parameters. QCA is objective but rarely used prospectively in clinical practice. A modified score (V-RESOLVE) has subsequently been developed, where the 4 angiographic variables are calculated by visual estimation13.
Although other variables may influence the risk of SB occlusion, and an individualised assessment should be performed, these scores can help in the risk stratification and identification of patients who need additional attention paid to SB protection.
The importance of the jailed wire technique: old and new evidence
Previous recommendations
The jailed wire technique consists of leaving a wire in the SB while implanting a stent in the MV (Central illustration). This manoeuvre has been recommended in previous EBC consensus documents14151617 as it has the following potential advantages:
– The technique helps to keep the SB open, and, in case of occlusion, the guidewire is the only marker of the SB ostium.
– It facilitates access to the SB by favourably changing the angle of the bifurcation.
– The jailed wire can provide anchoring of the guide catheter which facilitate balloon crossing through the stent struts to the SB.
– In case of SB occlusion, the jailed wire can be used to pass a low-profile balloon (or penetrative microcatheter) between the stent and the vessel wall to restore SB flow. The intervention can then be completed with the inverted crush or any other technique1819 (Figure 1).
These recommendations have been supported by the experience of expert operators and by some observational studies20. The risk of wire fracture is very low1221, and although cases of this have been described222324, the advantages of this technique outweigh the risks. Long jailed segments (>15-20 mm), especially in calcified vessels, should be avoided, but if significant resistance is encountered in attempted jailed wire removal, several techniques exist to ensure safe removal. Firstly, interaction between the guiding catheter and the proximal stent edge must be avoided, if ostial, through disengagement of the catheter from the artery. The guide and side branch wires may be withdrawn together while maintaining the main vessel and the unjailed side branch wire in place. Once the side branch wire is free, the guide catheter can be slowly readvanced over the existing two wires. If necessary, the passage of a balloon through the lumen of the main vessel stent can provide increased stability and allow more controlled retrograde traction on the jailed wire. Failing that, a balloon catheter can be used on the jailed wire to release contact with the main vessel stent, using the SB rescue technique25.
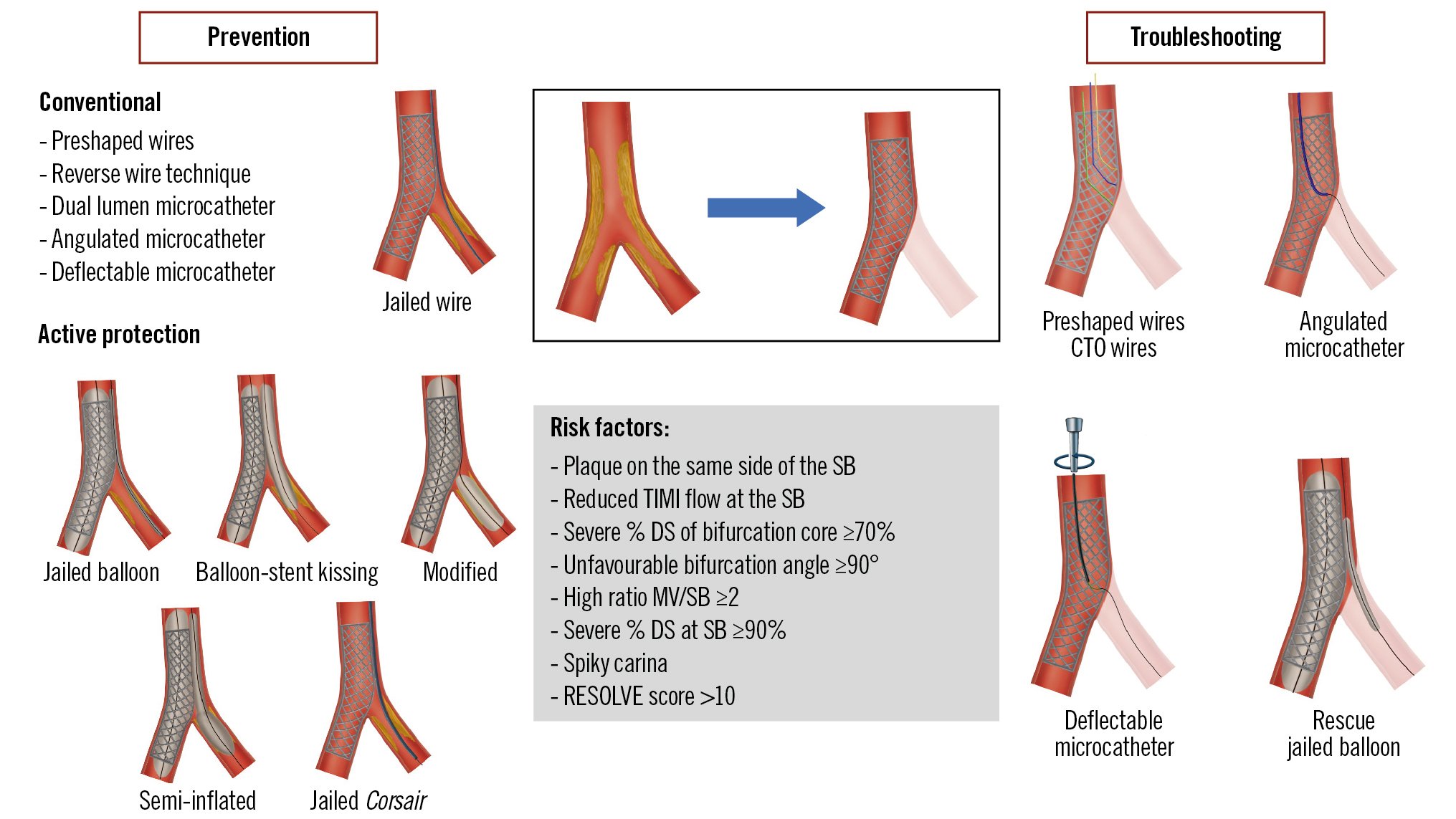
Central illustration. Preserving SB access during provisional stenting. CTO: chronic total occlusion; DS: diameter stenosis; MV: main vessel; RESOLVE: Risk prEdiction of Side branch OccLusion in coronary bifurcation intervention; SB: side branch; TIMI: Thrombolysis in Myocardial Infarction
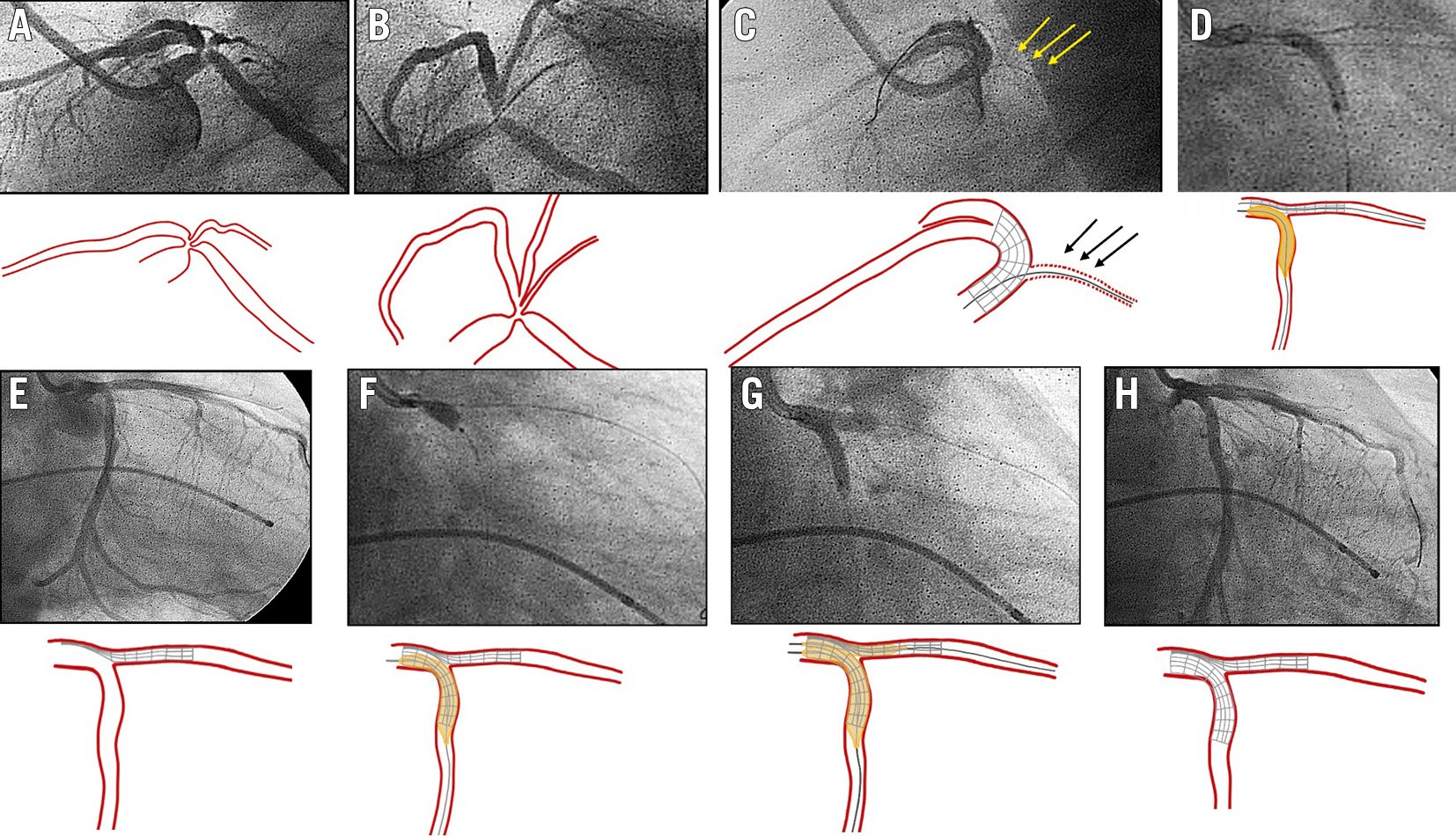
Figure 1. Inverted crush as a rescue technique for acute side branch occlusion. A, B) Baseline angiography, lateral (A) and spider (B) views, showing a severe bifurcation lesion at the distal LM (Medina 1,1,1). C) Acute LCx occlusion after LM-LAD stent implantation. Jailed guidewire at LCx (arrows). D) Abluminal inflation of a balloon at LCx advanced through the jailed wire (after previous low-profile balloon dilation). E) Patent LCx while the stent at the LAD remains crushed. F) DES implantation from LM to LCx artery (inverted crush technique). G) Rewiring of the LAD and final kissing balloon inflation. H) Final angiographic result. DES: drug-eluting stent; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main
New evidence
Recent publications have strengthened the previous recommendations for more complex bifurcation lesions. In a series of bifurcation-chronic total occlusion (CTO) lesions26 mainly treated with provisional stenting, the inability to protect the SB with a wire was linked to procedural failure and a higher incidence of periprocedural myocardial infarction. In the COBIS III Registry12, SB wire jailing before MV stenting was associated with a significantly lower rate of final SB occlusion in patients with stenosis at the SB and MV ≥60%, but not in overall bifurcation lesions. They did not therefore recommend routine SB wire jailing for all bifurcation lesions treated with the provisional stenting strategy, but only for complex bifurcations lesions with severe stenosis of the SB or MV. In the EBC MAIN Study27, failure to rewire a side vessel was more common when jailed wires were not used. Today, although there is consensus on the need for jailed wires, there is no definite consensus on the anatomies in which a jailed wire is essential. Some operators think that MV predilation hints at how the SB might behave after MB stenting. Accordingly, in cases of SB compromise after MV predilation, wiring of the SB should always be performed.
SB wiring in challenging anatomies
During provisional stenting, baseline SB wiring, as mentioned above, is recommended. After MV stenting and POT, SB rewiring can be required to perform a kissing balloon inflation (KBI) and/or to implant a second stent if there is severe stenosis or occlusion of the side branch origin17.
Baseline SB wiring in challenging anatomies
SB wiring can be challenging in certain anatomical conditions (Figure 2), such as extreme vessel tortuosity, extreme angulation of the origin of the SB in relation to the MV, or severe stenosis in both the SB and MV. In this context, it is crucial to select the optimal view to see each ostium of the bifurcation. Several techniques are available to address and overcome these situations (Central illustration):
1. Preshape the tip of the wire according to the bifurcation anatomy. The curves typically used for this purpose are: 1) a single bend with a short (2-3 mm) tip, 2) a single bend with a long (4-6 mm) tip, 3) a single wide-angled bend and 4) a double-bend shape28. If SB wiring using these curves fails, the operator can use more sophisticated techniques which are described below.
2. The reverse wire technique: for this technique, two sharp curves are created at the guidewire tip: a long proximal one, which should create a loop in the distal MV and an opposite short distal one, which should engage the SB ostium during the wire pullback29. This technique can be facilitated using a dual lumen or angulated microcatheter.
3. Dual lumen microcatheters. A dual lumen microcatheter is particularly useful for a bifurcated CTO lesion (Figure 3), a tortuous SB ostial lesion, and tricky bifurcation angles (Figure 2), which require back-up force and directional SB wiring. Several models are currently available (Table 1). The external diameter at the tip and at the distal shaft, as well as the distance between the tip and the over-the-wire (OTW) lumen exit port are variable among the different models (Table 1). For SB wiring, the MV wire is introduced into the monorail lumen, and the whole catheter is advanced up to the level of the SB ostium. The OTW exit port should be adequately orientated facing the SB origin, thus facilitating the passage of a second wire towards the SB. Sometimes, the OTW exit port becomes misoriented, and the operator must retrieve the microcatheter with the SB wire inside, try to rotate the system and then advance it again, checking the location of the OTW exit port. This manoeuvre can be repeated until the right position is achieved. These microcatheters are especially useful in coronary bifurcation lesions associated with a CTO (Figure 3), as they facilitate wire passage through long segments of dissection, without risk of subintimal dissection extension (Figure 3). It is important to note that the use of a dual lumen microcatheter alters the properties of the selected guidewire, increasing the penetrative force through added support. Operators should therefore be aware of the risk of vessel injury.
4. Dedicated single lumen angulated microcatheters. The SuperCross Angled Tip Microcatheter (Teleflex) is available in 130 and 150 cm lengths with different tip angles. The 120° model is the most frequently used for extreme SB angulations30 (Figure 4). The recommended steps for SB access are:
– Deliver the SuperCross distally to the bifurcation over the MV wire,
– Pull back the wire within the SuperCross,
– Pull back the SuperCross while torquing the tip towards the SB origin,
– Re-advance a new wire to cross the SB ostium.
This technique may be easier in Medina X,0,X bifurcation lesions where the SuperCross may be rotated at the level of the distal MV. On the contrary, in Medina X,1,X bifurcation lesions, the orientation of the SuperCross in front of the SB origin can be more complicated because of a lack of space at the level of the SB ostium. These devices should be managed with care to avoid the possibility of wall damage/dissection while the dispositive is being rotated.
5. Deflectable tip single lumen microcatheters. The Venture (Teleflex) wire-control catheter has a radiopaque 8 mm distal tip that can be deflected up to 90º with a clockwise torque shaft in the proximal part of the catheter. The catheter is advanced to the bifurcation lesion in its straight configuration along the guidewire. Once the target is reached, the tip is deflected to the desired angle to access the SB ostium. Once the guidewire has been advanced into the SB, the torque shaft may be turned anticlockwise to straighten the tip before withdrawing the catheter. The success rate of this device, following failure of conventional techniques, is approximately 85%3132.
6. The balloon block and support technique. This technique is not generally recommended but can be used if dedicated single or dual microcatheters are unavailable. In cases with significant angulation between the SB and MV, the SB guidewire usually prolapses into the distal MV instead of the SB despite an appropriate tip shape. To mitigate this risk, an uninflated balloon may be placed in the distal MV, close to the carina. Once the tip of the SB guidewire reaches the level of the SB ostium, the MV balloon is inflated to temporarily block the distal MV facilitating the advancement of the guidewire into the SB33.
7. MV balloon predilation. This is considered the “last resort” strategy to wire the SB28. Indeed, this technique is not advisable in the vast majority of bifurcation lesions as it may cause plaque and carina shift, ultimately resulting in SB occlusion. Nevertheless, MV balloon predilation with a small balloon can be considered in highly selected bifurcation lesions when all other options have failed. Unfavourable anatomies, such as those with large plaque burden in both the MV and SB and a wide angle, may prevent the advancement of any appropriately curved wire or device at the bifurcation lesion site. In this situation, gentle predilation of the proximal MV may create enough space to successfully advance and manipulate an angulated catheter or an appropriately shaped wire toward the SB. In case of severely calcified lesions, MV rotational atherectomy may be a good alternative to MV balloon predilation. In a bifurcation subgroup analysis of the PREPARE-CALC Trial, rotational atherectomy in the MV resulted in fewer compromised SBs at the end of the procedure, as compared with a strategy of MV balloon dilatation using a scoring/cutting balloon34.
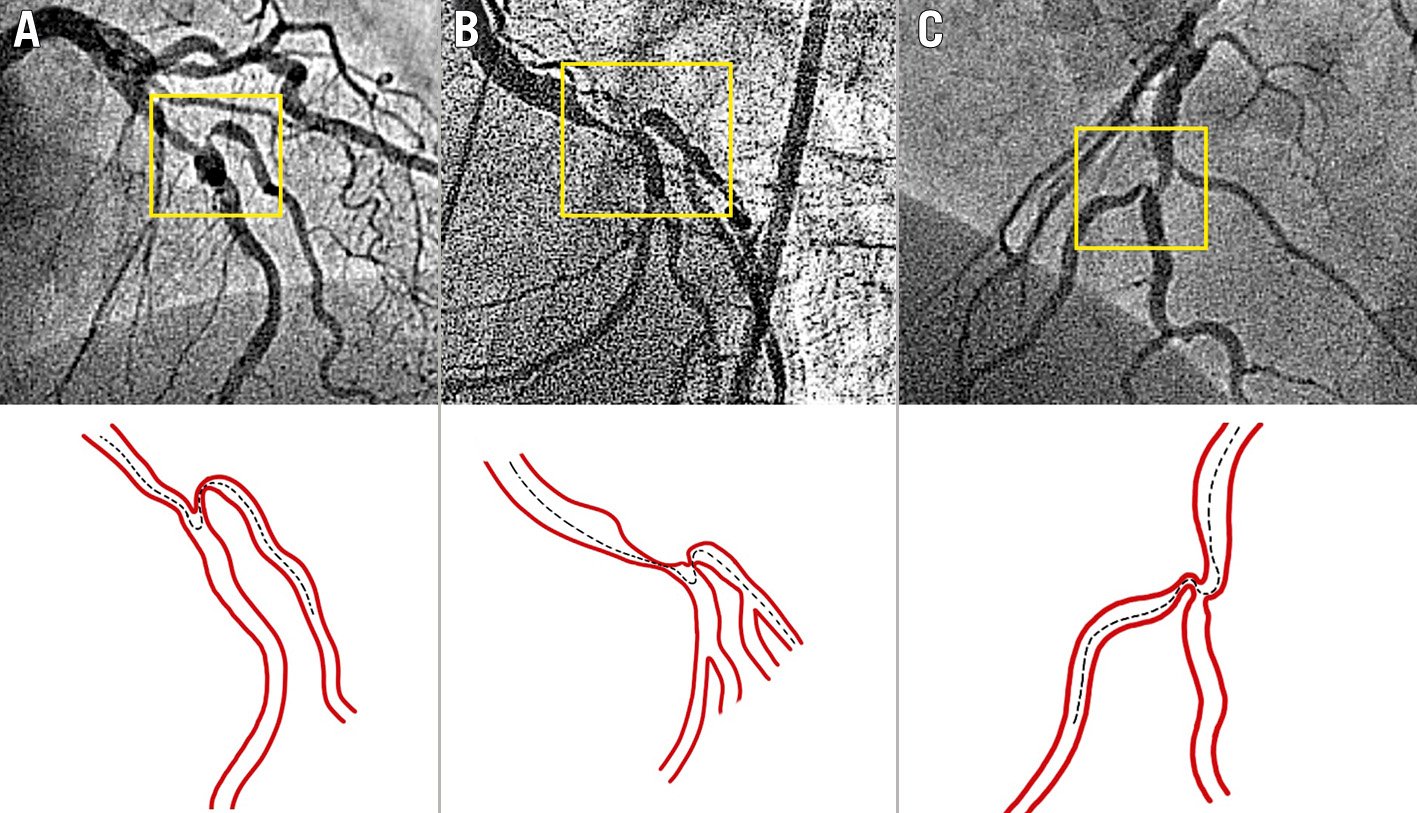
Figure 2. Examples of difficult SB wiring in bifurcation lesions due to severe stenoses and unfavourable angulation of the SB origin in relation to the MV. A and C) LAD/diagonal branch. B) LCx/obtuse marginal. The dotted lines show the theoretical trajectory of the wire. Appropriately preshaped wire tips or any of the techniques described in the text will be needed to wire the MV and SB. LAD: left anterior descending artery; LCx: left circumflex artery; MV: main vessel; SB: side branch
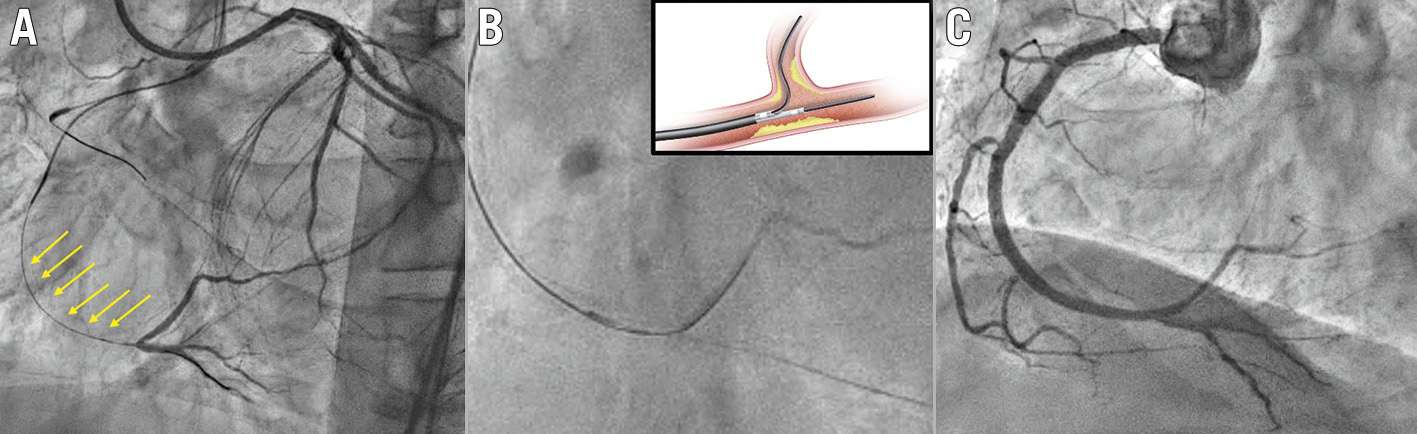
Figure 3. Dual lumen microcatheter in bifurcation lesions associated with CTO. Example of a right coronary artery CTO with a bifurcation at the distal cap and a long proximal dissected segment (arrows). A) Wire into the true lumen of the posterior descending artery. B) Dual lumen microcatheter to pass a new guidewire into the posterolateral branch. C) Final angiographic result after provisional stenting. CTO: coronary chronic total occlusion
Table 1. Dual lumen microcatheters.
| Name | Manufacturer | Usable length, cm | Outer diameter, tip (Fr) | Distance to radiopaque marker, mm | Outer diameter, distal shaft (Fr) | Outer diameter, proximal shaft (Fr) | Tip to OTW port distance, mm | Tip to Rx port distance, mm |
|---|---|---|---|---|---|---|---|---|
| FineDuo | Terumo | 140 | 1.4 | 0.5 | 2.9 | 3.2 | 6.5 | 210 |
| Sasuke | Asahi | 145 | 1.5 | 4 | 2.5 x 3.3 | 3.2 | 6.5 | 200 |
| Twin-Pass | Teleflex | 135 | 2.0 | 1 & 20 | 2.7 x 3.4 | 2.9 | 20 | 210 |
| Twin-Pass Torque | Teleflex | 135 | 2.1 | 1 & 7 | 3.5 | 3.1 | 7 (10º) | 220 |
| NHancer Rx dual | IMDS | 135 | 1.5 | Tip & 6.5 | 2.3 x 3.3 | 2.6 | 6.5 | 180 |
| ReCross Dual | IMDS | 140 | 1.5 | Tip, 8 & 12 | 2.3 x 3.3 | 2.6 x 3.4 | 8 &12 (opposite directions) | Only OTW |
| Crusade | Kaneka | 140 | 2.2 | 1 | 2.9 | 3.2 | 6.5 | 210 |
| OTW: over-the-wire; Rx: rapid exchange | ||||||||
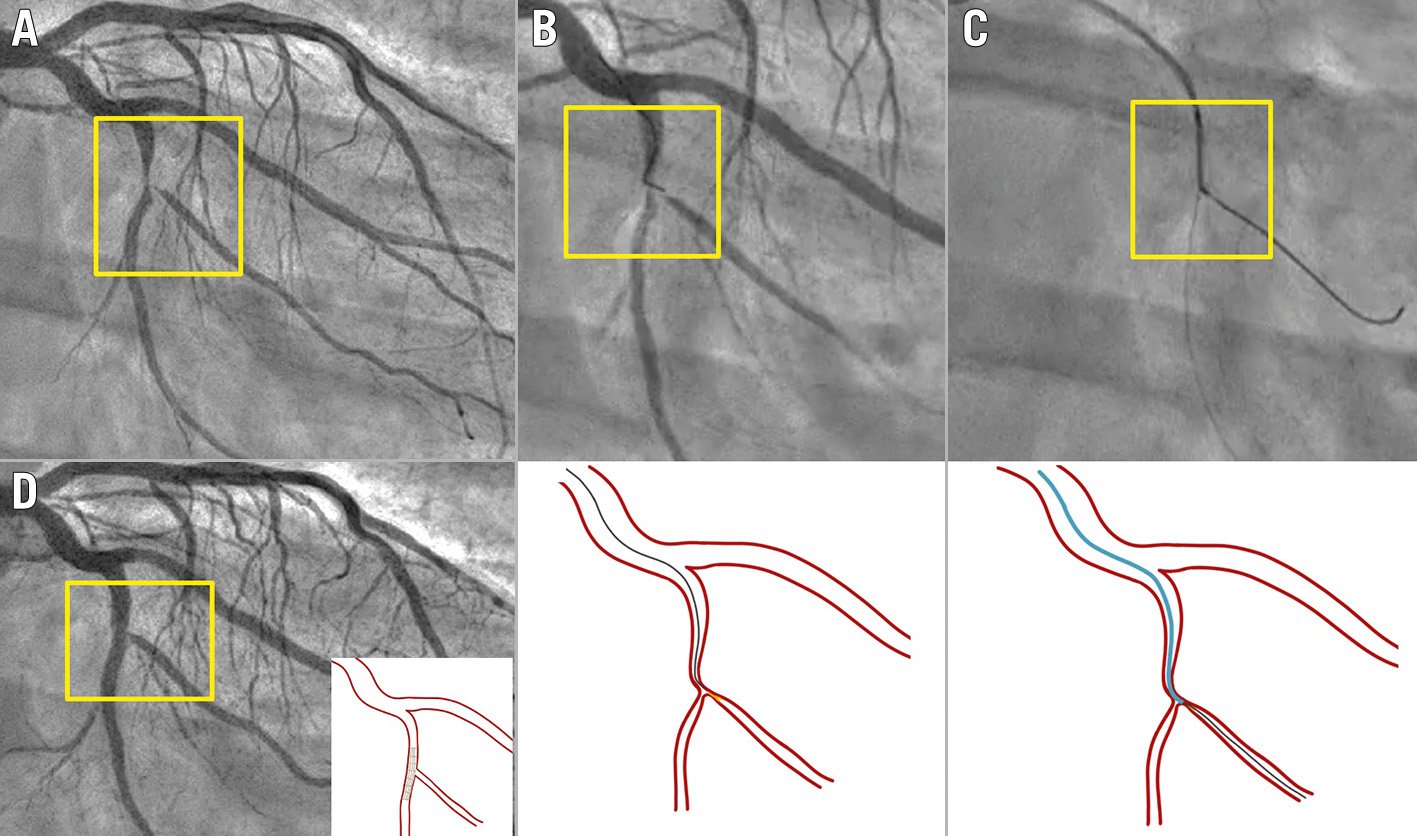
Figure 4. Angulated microcatheter in difficult baseline SB wiring. Severe bifurcation lesion involving the left circumflex artery/marginal branch (Medina 1,1,1). A) Baseline angiography. B) Failure to cross with preshaped conventional wire. C) An angulated microcatheter (SuperCross 120º) was placed distally to the bifurcation, and after a pull back and smooth torquing, the tip was oriented towards the SB origin, facilitating the wiring. D) Final result after provisional stenting. SB: side branch
SB rewiring after MV stenting in challenging anatomies
In the provisional stenting approach, further interventions to the SB may be required following MV stent implantation. SB rewiring can be technically complex because of the baseline anatomy and/or severity of the stenosis at the SB origin after MV stenting. The most challenging situation is occlusion of a severely angulated SB.
SB rewiring is facilitated by some preliminary manoeuvres
– Leave a wire in the SB during MV stent implantation (jailed wire technique).
– POT should be performed before SB rewiring to reduce the risk of accidental wiring outside the stent in the main vessel. This manoeuvre should be performed in accordance with the EBC’s previous recommendations1735. A too distal position of the balloon when the bifurcation is not perfectly visualised can cause SB occlusion.
– Consider inverted provisional stenting when SB access is more difficult than MV access.
– SB balloon predilation before MV stenting. This approach is not generally recommended, but it may be useful in a severely calcified or angulated side branch stenosis, or when access to the side branch is difficult, or when side branch flow is impaired after wiring35. This strategy could have the following advantages3637: a) it could increase the ostial lumen and, thus, facilitate rewiring of the SB; b) it could help to maintain side branch flow after MV stent implantation; c) it could be a definitive procedure on the SB and avoid distortion of the main vessel stent. However, side branch dissection is more common with this technique27 and may prevent rewiring and lead to adverse outcomes.
SB rewiring through the MV stent struts
Most of the techniques described above may be useful in this situation, with some variations (Central illustration).
1. Guidewire tip configuration for crossing the MV stent side cells, as well as the type of wire, depends on the preferences of the operator. A single wide-angled bend or a double-bend shape seem to be the most popular options for this purpose. Pulling back the wire from the distal MV and allowing it to drop into the SB increases the success rate and the chance of distal strut crossing.
2. The reverse wire technique is less useful for SB rewiring than for initial SB wiring. In contrast, angulated and deflectable tip catheters may be more effective than they are in baseline conditions (Figure 5, Figure 6). The large MV lumen created by the stent allows the rotation and manipulation of these catheters to direct their tip toward the SB ostium. At the same time, they provide good support if there is resistance crossing the SB ostial stenosis/occlusion. Dual lumen microcatheters are also useful in this context to avoid abluminal wire crossing17 and facilitate SB wiring.
3. If an important SB becomes occluded and side branch wiring attempts are not successful, an initial re-POT should be undertaken to ensure full stent strut expansion, followed by further attempts to recross. If, however, these strategies fail, a “rescue” balloon can be advanced behind the stent along the jailed wire into the side vessel1838 (Figure 1). As an alternative, a Corsair (ASAHI) catheter can be used for this purpose (Figure 7). Depending on the ability to maintain SB patency and to rewire the side branch with patency restored, the procedure may progress to a stepwise provisional stenting strategy (with T and small protrusion [TAP] technique if needed) (Figure 7), or an inverted crush technique (with or without double-kissing). The rewiring to the dissected branch along the jailed balloon (Real JAB) technique presented by Kinosita at the 2022 EBC meeting39 is a modification of the above-described “rescue” balloon technique. It was designed to ensure wire insertion into the true lumen of a dissected SB. It consists of inflating the jailed balloon at the true lumen of the SB, while a second wire is inserted into the SB through the MV stent struts. Immediately after balloon deflation, the wire is advanced to try and reach the SB true lumen.
4. The use of dedicated CTO wires provides another rescue strategy for SB rewiring after SB occlusion, although with a higher risk of vessel injury and subintimal tracking with risk of vessel closure.
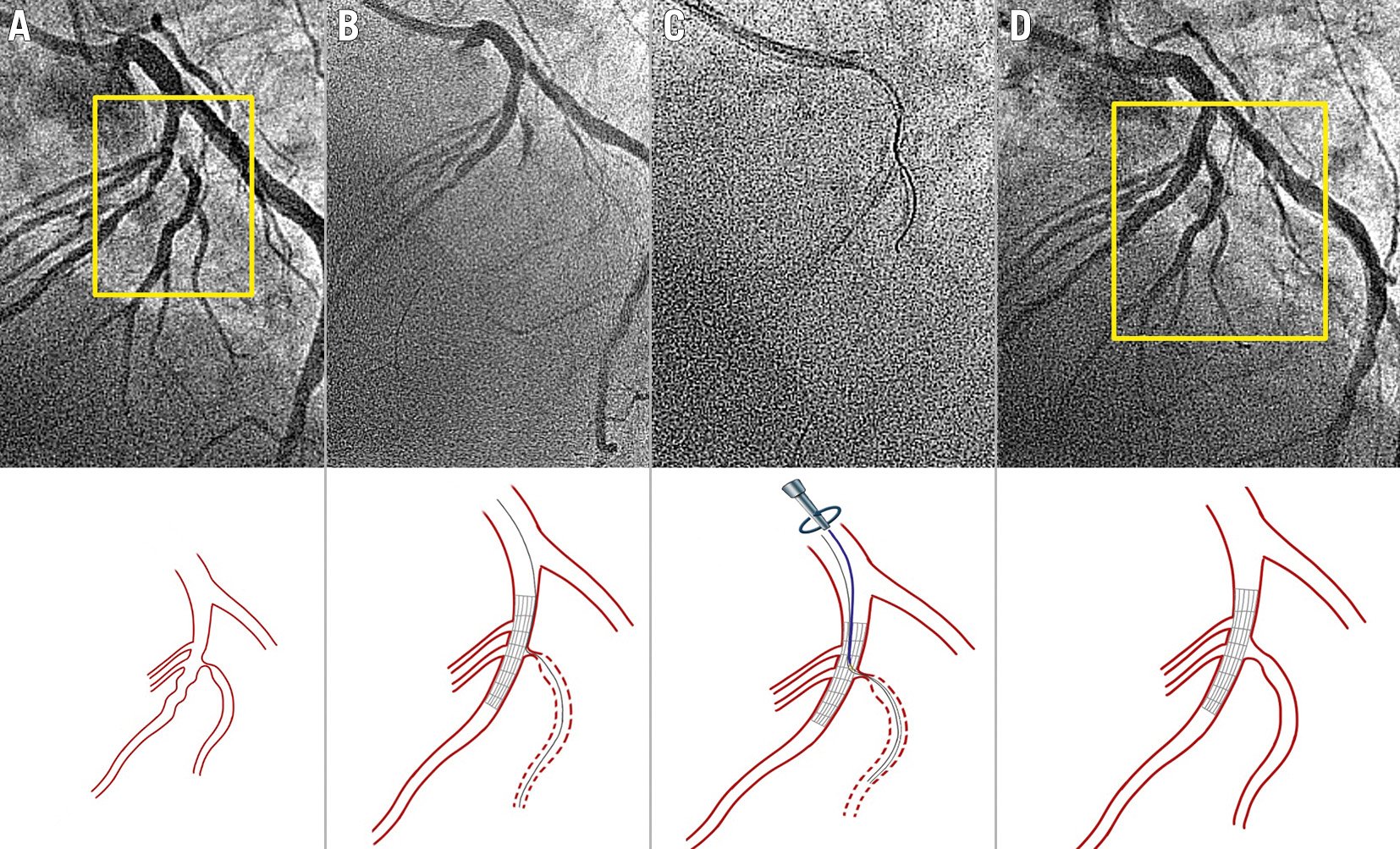
Figure 5. Deflectable tip catheter for occluded SB rewiring after MV stenting. A) Bifurcation lesion with a critical stenosis at the diagonal branch origin. B) SB occlusion after main vessel stent implantation with the jailed wire at the diagonal branch. C) The Venture catheter was properly oriented and deflected to facilitate the SB rewiring. D) Final result after provisional stenting. MV: main vessel; SB: side branch
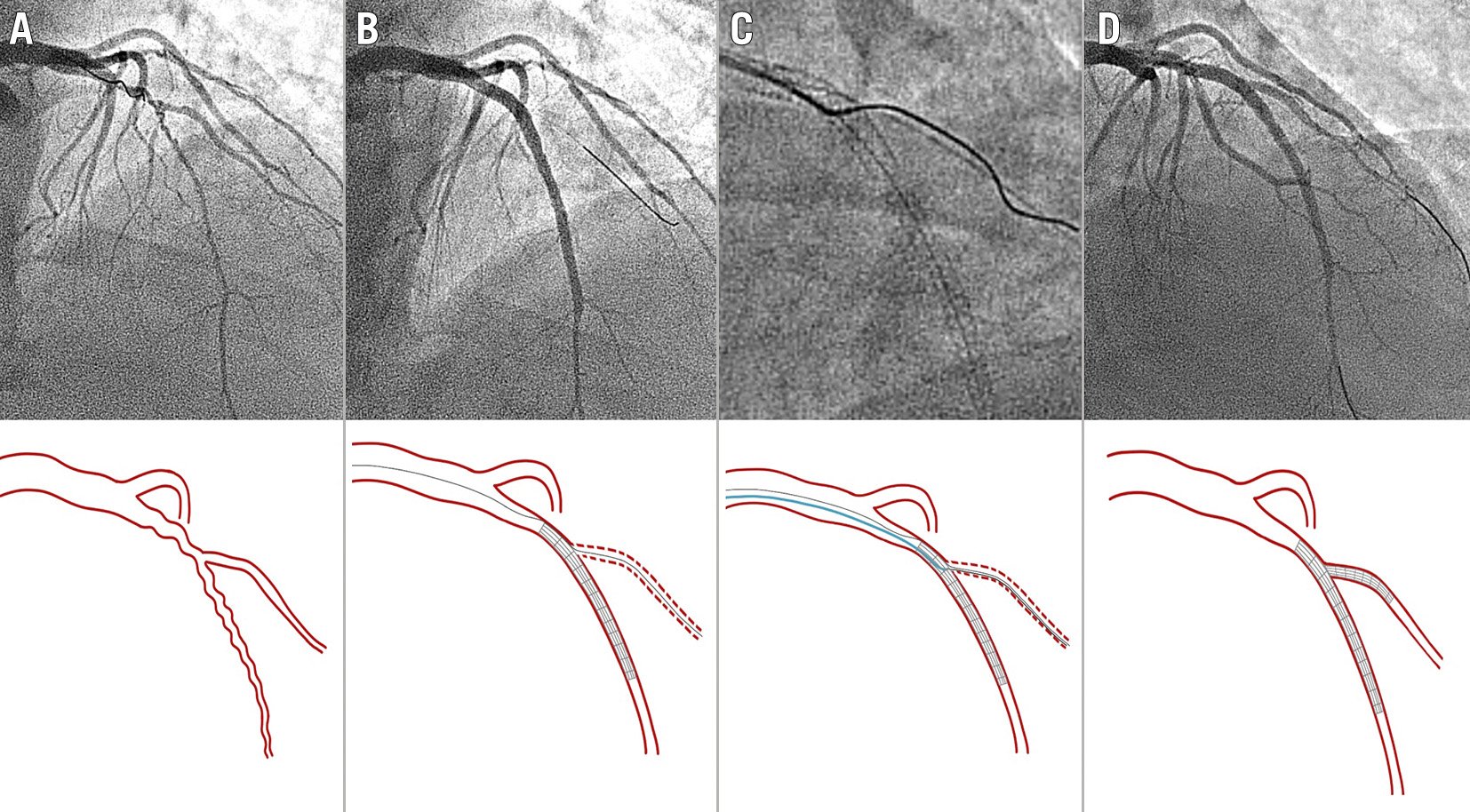
Figure 6. Diffuse lesion at the LAD involving a diagonal branch (Medina 1,1,0). A) Baseline angiography. B) Acute SB occlusion after LAD stent implantation (jailed wire at diagonal branch). C) The microcatheter can be more easily manipulated and oriented than in the example of Figure 4 because the MV distal segment is wider (already stented). D) Final angiographic result after stent implantation at diagonal branch and final kissing balloon. LAD: left anterior descending artery; MV: main vessel; SB: side branch
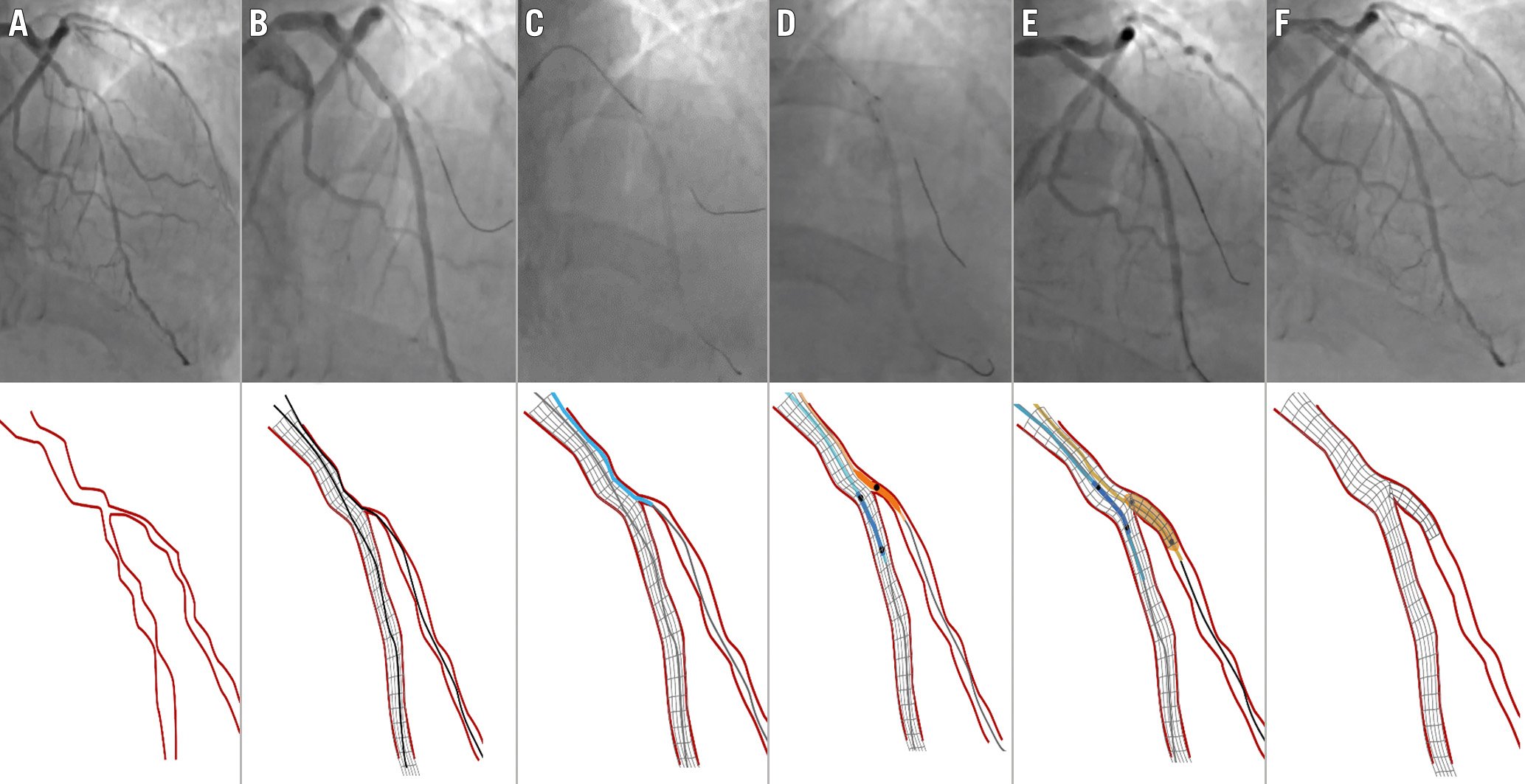
Figure 7. Corsair catheter used for SB rescue following the unsuccessful passage of a small balloon on a jailed wire behind the MV stent. A) Baseline anatomy LAD/D1 (Medina 1,1,1). B) D1 occlusion after LAD stenting. A small balloon cannot cross behind the stent through the jailed wire. C) A Corsair microcatheter crosses behind the stent on the jailed wire. D) Small balloon on a jailed SB wire and protective balloon inside the stent. E) TAP stenting. F) Final result after new SB rewiring, TAP and kissing balloon inflation. D1: first diagonal; MV: main vessel; LAD: left anterior descending artery. TAP: T and protrusion
SB jailed balloon technique and active SB protection
The jailed wire technique is known to reduce but not eliminate the risk of transient or persistent SB occlusion. For this reason, the jailed balloon technique was developed and first described by Burzotta et al40. This technique involves the placement of an uninflated balloon in the SB during MV stenting. The uninflated balloon remains jailed under the MV stent after its deployment, reducing both carina and plaque shifts due to its presence. If the SB flow is preserved after MV stenting, the jailed balloon is removed, uninflated. If the SB is occluded after MV stenting, the SB jailed balloon is inflated to restore the flow in the SB.
Following the original description40, additional modifications have been reported41 (Central illustration), including: 1) balloon-stent kissing technique4243; 2) modified jailed balloon technique4445; 3) semi-inflated balloon technique46; 4) double-kissing inflation outside the stent47; 5) jailed Corsair technique4849. The balloon-stent kissing technique requires a balloon with adequate length that is appropriately sized, to approximate or be smaller than the SB reference vessel diameter, to be advanced into the SB. The MV stent is then advanced to the correct position at the bifurcation site. The jailed SB balloon is inflated first at low pressure (6-8 atm), while the MV stent is deployed at nominal pressure. The SB balloon is then deflated and removed, leaving the wire in the SB. Finally, the stent balloon is fully inflated to correct any stent deformation, per the conventional technique. At this point, the procedure continues per the conventional provisional stenting strategy. None of the four subtle variations mentioned above44454647 have demonstrated superiority over the balloon-stent kissing technique.
Observational studies have shown that all these modalities of jailed SB balloon techniques for complex, true bifurcation lesions offer good, immediate procedural success, with excellent SB protection that prevents SB occlusion during the procedure, and with good immediate clinical outcome in patients treated with the provisional stenting approach. Table 2 describes SB protection and rescue techniques.
Although the jailed SB balloon technique has demonstrated its efficacy in protecting the SB in complex bifurcations treated by provisional stenting, the standard jailed SB wire technique is easier to perform. The two techniques have been compared in a randomised trial. The CIT-RESOLVE Trial50 included 335 patients with high risk of SB occlusion randomised to either the conventional strategy with the jailed SB wire technique or the SB protection strategy with the jailed SB balloon. The study demonstrated that the active SB protection strategy was superior to the conventional strategy and was associated with a significantly lower incidence of SB occlusion and loss of SB flow immediately after full apposition of the MV stent. However, a subgroup analysis demonstrated no significant difference in major adverse cardiac events (MACE) at 1-year follow-up51. A meta-analysis comparing active versus conventional SB protection has been recently published52. This analysis consisted of 1,174 patients with bifurcation lesions treated with either strategy. The authors concluded that the active SB protection strategy in bifurcation lesions was associated with reduced SB deterioration, but it did not decrease procedural myocardial infarction or improve the long-term prognosis.
Table 2. Side branch protection and rescue techniques.
| TECHNIQUE | WHEN TO APPLY | DESCRIPTION |
|---|---|---|
| Jailed wire | Before MV stenting | Wire placement in the SB |
| Jailed balloon protection | Before MV stenting | Small-diameter balloon placed in the SB and kept uninflated during MV stent deployment |
| Jailed microcatheter (including jailed Corsair) | Before MV stenting | Microcatheter placed in the SB and kept uninflated during MV stent deployment |
| Inflated jailed balloon protection (including modified jailed balloon and balloon-stent kissing) | Before MV stenting | Small-diameter balloon (with different degrees of protrusion in the MV) placed in the SB and kept inflated during MV stent deployment |
| Semi-inflated jailed balloon protection | Before MV stenting | Small-diameter balloon placed in the SB and inflated at low atmospheres during MV stent deployment |
| Rescue balloon jailing | After MV stenting, in the case of SB occlusion (or jailed wire entrapment) | Small-diameter balloon advancement and inflation over the jailed wire |
| Rescue microcatheter jailing | After MV stenting, in the case of SB occlusion (or jailed wire entrapment) | High-penetration microcatheter advancement over the jailed wire |
| MV: main vessel; SB: side branch | ||
Conclusions
Stepwise provisional stenting can be adopted for most complex bifurcation lesions. However, side branch occlusion after main vessel stenting can occur and is not always straightforward to fix. Use of angiographic and intracoronary imaging criteria to predict the risk of side branch closure may encourage use of side branch protection strategies. These protection approaches have been discussed and developed following several EBC meetings and provide strategies to avoid SB closure or overcome compromise following MV stenting, minimising periprocedural injury and ensuring a durable outcome for patients with bifurcation coronary lesions.
Conflict of interest statement
M.Pan has received speaker fees from Abbott, Terumo, and Volcano. J.F.Lassen has received speaker fees from Medtronic, Boston Scientific, Biotronik, Abbott, and Biosensors. F.Burzotta has received speaker fees from Medtronic, Abiomed, Abbott, and Terumo. S.Ojeda has received consulting fees from Medtronic and Edwards Lifesciences; and speaker fees from Philips and World Medical. R.Albiero has received speaker fees from Medtronic and Abbott. T.Lefèvre has received speaker or proctorship fees from Abbott, Boston Scientific, Terumo, and Edwards Lifesciences. D.Hildick-Smith disclosed advisory board/consultancy/research funding from Terumo, Medtronic, Abbott, and Boston Scientific. T.W.Johnson has received speaker fees from Abbott, Boston Scientific, Medtronic, and Terumo; and institutional funding for fellowships from Boston Scientific and Terumo. A.Chieffo has received speaker fees from Abiomed and GADA.A.P.Banning received institutional funding of afellowship from Boston Scientific; and has received speaker fees from Boston Scientific, Abbott, Medtronic, Philips/Volcano, and Miracor. O.Darremont has received speaker fees from Edwards Lifesciences. Y.S.Chatzizisis has received speaker fees/consultancy/research funding from Boston Scientific and Medtronic. G.Stankovic has received speaker fees from Medtronic, Abbott, Boston Scientific, and Terumo. The other authors have no conflicts of interest to declare.

