Abstract
Aims: Provisional stenting with drug-eluting-stents is actually adopted to treat most of bifurcated lesions. A major drawback of this technique is the risk of side-branch (SB) closure after main vessel (MV) stenting.
Methods and results: We set-up, and bench tested, a novel technique for SB protection based on the placement of a balloon in the SB during MV stenting. The uninflated balloon, which remains jailed under the stent struts, serves to reduce both carina and plaque shifts due to its SB ostium spatial occupation. If SB flow is preserved after MV stenting, the jailed balloon is removed uninflated. If the SB becomes occluded after MV stenting, the jailed balloon may either be used as a marker and a favourable angle modifier to facilitate rewiring or can be dilated to try to restore SB flow. SB rewiring and kissing balloon inflation must be performed to correct stent deformation or malapposition. This novel technique has been successfully adopted in 20 patients with complex (55% unprotected left main, 85% Medina 1,1,1 lesions) true bifurcated lesions undergoing drug-eluting-stent implantation.
Conclusions: The jailed balloon protection is a novel technique aimed at improving SB protection during provisional stenting of bifurcated lesions considered at high risk of SB compromise after MV stenting.
Introduction
Simple main vessel (MV) stenting with “provisional” stenting of the side-branch (SB)1 is the most commonly adopted strategy2-3 to treat bifurcated lesions but is associated to the risk of SB closure after MV stent implantation. The placement of a second wire in the SB during MV stenting (“jailed wire”) is known to reduce the risk of transient or persistent SB occlusion4, but is not able to abolish it.
In the present manuscript we present a new technique for SB protection during MV stenting based on the use of a “jailed balloon”.
Materials and methods
Technique’s description and bench testing
In vitro testing of the technique was performed under fluoroscopic control using a silicon phantom of coronary bifurcation (tube lumen diameters: 4 mm proximal MV, 3.5 mm distal MV, 2.5 mm SB). After wiring both MV and SB, a 3.5x18 mm slotted-tube drug-eluting stent was placed in the MV and a 2x20 mm semi-compliant coronary balloon was placed in the SB (Figure 1). Then, the stent was implanted in the MV with the uninflated balloon kept in the SB (“jailed” under the MV stent struts) (Figure 1). Thereafter, two situations after MV stenting were simulated: SB flow persistence and SB occlusion. In the first situation the jailed balloon was removed uninflated without evidence of major MV stent distortion or vessel wall malapposition (Figure 1). It is noteworthy to recognise that the difficulty experienced during jailed balloon removal was comparable to the one felt when a jailed wire is removed.
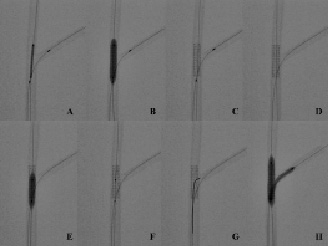
Figure 1. Bench testing of the jailed balloon technique without side-branch balloon inflation. (A) Stent (3.5x18 mm) positioning in the main vessel (MV) with protection balloon (2x20 mm) into the side-branch (SB). The balloon is placed with the proximal marker immediately proximal to the stent. (B) Deployment of MV stent with jailed balloon. (C) MV stent appearance after deployment and before jailed balloon removal. (D) MV stent appearance after uninflated jailed balloon removal. No major stent deformation is detectable. (E) Proximal MV post-dilation with short (4x12 mm) balloon. (F) MV stent appearance after post-dilation of the proximal portion. (G) SB rewiring is performed using a pullback technique from distal to proximal so that MV stent’s side-cells are re-crossed in the distal part of the SB ostium. (H) Kissing balloon inflation (3.5x20 mm in the MV and 2.5x20 mm in the SB).
In the second situation (i.e., SB occlusion), the SB balloon was inflated to simulate attempt to reopen the SB (Figure 2). After deflation, the balloon was removed leaving an appreciable distortion of the stent struts and a gap between stent struts and vessel wall in the MV proximally to the SB take-off (Figure 2). To ensure prompt correction of stent distortion and malapposition, a short balloon (semi-compliant 4x12 mm) had been placed in the MV before jailed SB balloon inflation and was inflated immediately after jailed SB balloon removal. This post-dilation was able to completely re-appose the MV stent struts to the vessel wall (Figure 2). In both situations the procedure was completed with the standard kissing balloon technique. Thus, SB rewiring was performed (using a pullback technique from distal MV to proximal with the aim of crossing the distal side cells of the MV stent) and final kissing balloon inflation was performed with semi-compliant 3.5x20 mm balloon in the MV and 2.5x20 mm in the SB. In both cases, the final result was identical to that obtained with the standard jailed guidewire technique supporting the hypothesis that final kissing balloon inflation is able to completely correct the variable degrees of MV stent distortion associated with intermediate phases of the jailed balloon technique (see Figure 3 for comparison of angiographic appearance obtained in bench tests with uninflated jailed balloon technique, inflated balloon technique and standard jailed guidewire technique). The absence of post-procedural stent malapposition in both uninflated jailed balloon and inflated balloon technique was confirmed by intravascular ultrasound assessment (Figure 4).
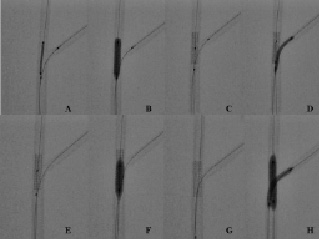
Figure 2. Bench testing of the jailed balloon technique with side-branch balloon inflation. (A) Stent (3.5x18 mm) positioning in the main vessel (MV) with protection balloon (2x20 mm) into the side-branch (SB). The balloon is placed with the proximal marker immediately proximal to the stent. (B) Deployment of MV stent with jailed balloon. (C) Before inflating the SB balloon, the short (4x12 mm) balloon necessary for proximal MV post-dilation is placed in the MV. (D) SB balloon is removed. (E) The stent has a detectable deformation in the proximal part which has been induced by SB balloon dilation. (F) Proximal MV post-dilation with the short (4x12 mm) balloon. (G) MV stent appearance after post-dilation of the proximal portion. The MV stent distortion is completely corrected so that SB rewiring is performed as usual (H) Kissing balloon inflation (3.5x20 mm in the MV and 2.5x20 mm in the SB).
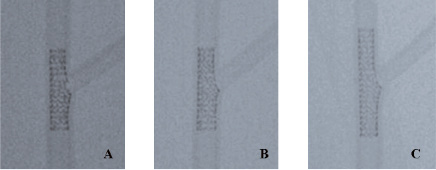
Figure 3. Final appearance of jailed balloon technique without balloon inflation, with balloon inflation compared with standard provisional stenting with jailed guidewire. The figure shows the angiographic appearance of the MV proximal to bifurcation obtained with jailed balloon technique without balloon inflation (A), with balloon inflation (B) and with standard provisional stenting with kissing inflation (C) obtained using a 3.5x23 mm Xience stent.
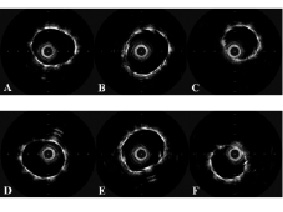
Figure 4. Final appearance of the jailed balloon technique with and without balloon inflation as assessed by intravascular ultrasound in the phantom model. The figure shows the intravascular ultrasound images obtained in the phantom model by automatic pullback in the main vessel. The images shows the post-procedural IVUS appearance of MV proximal to bifurcation (A,D), at the proximal level of bifurcation (B,E) and distal to bifurcation obtained (C,F) with jailed balloon technique without balloon inflation (A,B,C) or with balloon inflation (D-E-F).
Clinical case series
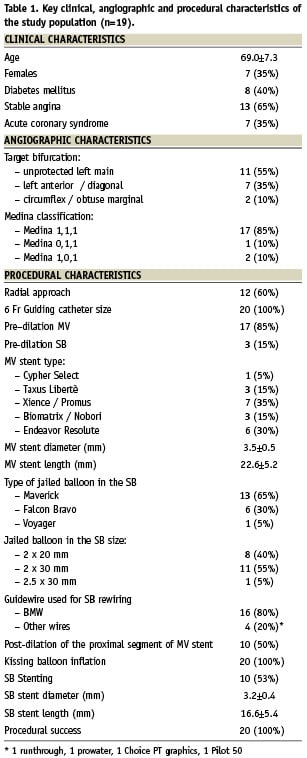
The jailed balloon technique has been successfully used in 20 patients with true bifurcated lesions undergoing provisional stenting with drug-eluting stents. The technique has been applied at the operators’ discretion when, on the basis of the angiographic appearance, the risk of flow deterioration was considered high in a relevant SB. The main clinical, angiographic and procedural characteristics of the treated patients are reported in the Table 1. The balloon selected for SB protection was mainly 2 mm in diameter and 20-30 mm in length (length selected to exceed the proximal edge of the MV stent with the proximal part of the SB balloon). In 17/20 patients SB flow was maintained after MV stenting so that the balloon was removed uninflated. SB rewiring was successfully performed either before jailed balloon removal or after uninflated SB balloon removal and kissing balloon inflation was performed with semi-compliant balloons. In three patients, SB occlusion after MV stenting was noted. In two of them an attempt to rewire the SB was successfully performed (within two minutes) using the uninflated jailed balloon as marker of SB location. In the last patient treated on a last remaining vessel bifurcated lesion, the occluded SB caused haemodynamic compromise so that the jailed balloon was inflated and then removed obtaining immediate SB flow restoration. No angiographic complication (nor any deterioration of the SB flow) was noted during balloon jailing or removal. Final kissing inflation was always successfully performed and in 10 patients a stent was implanted in the SB using the TAP-stenting technique5.
Discussion
Provisional T-stenting is the recommended approach to treat most of bifurcated lesions2, but it is associated with the risk of SB compromise after MV stenting. Known mechanisms of SB deterioration after MV stenting are plaque shift6,7 and carina shift8. If occlusion occurs, rewiring is attempted trying to reopen the SB9, but prolonged flow impairment in its territory may cause peri-procedural myonecrosis or (in high-risk subsets of patients) haemodynamic compromise. Nevertheless, impaired SB salvage is not successful in all the cases as testified by the 1.1% rate of SB occlusion observed in a recent trial10.
The present modification of the jailed guidewire technique is aimed at improving the safety of the provisional approach when the risk of SB impairment is anticipated to be high and the SB is considered relevant (large territory supplied). We speculated that a jailed balloon in the SB during MV stenting might provide a useful tool to reduce plaque/carina shift due to its higher occupation of the SB ostium. If SB flow is not impaired after MV stent implantation, the jailed balloon may be removed uninflated experiencing a difficulty which is, in both bench tests and in the first clinical experience, comparable to that encountered during jailed wire removal. The bench test suggested that the MV stent does not present a major distortion after uninflated jailed balloon removal. Nevertheless, as the creation of minor stent malappositions in the proximal MV segment cannot be excluded, in the clinical practice we used one or a combination of such tricks before jailed balloon removal: 1. advancement of the guidewire for SB rewiring in the distal MV, 2. rewiring of the SB, 3. placement of a balloon for proximal MV post-dilation.
In the case of SB occlusion, the jailed balloon could offer a more visible marker of the occluded SB as well as a bifurcation-angle modifier which may facilitate rewiring. If the attempt to rewire is not successful in a reliable time (1-2 minutes), the jailed balloon may be inflated to try to reopen the SB. This latter possibility was tested in a bench model showing that a detectable MV stent distortion is induced. Such distortion may create an obstacle to the advancement of a new guidewire for SB rewiring so that a balloon for MV post-dilation should be prepared before jailed balloon inflation to promptly re-appose the proximal MV stent struts. Furthermore, if jailed balloon inflation is not able to reopen the SB or creates major dissection, the space created by SB balloon dilation may theoretically be used for SB stenting according to an inverted “provisional crush” technique. All these possibilities should actually be considered speculative, as in our case series, jailed balloon inflation was performed only once.
The major concerns about the jailed balloon technique are related to both the possible risk of balloon entrapment into the SB and to distortion/malapposition to the MV stent struts.
The first issue, although theoretically relevant, was challenged by this preliminary experience, as no balloon rupture was observed, and the jailed balloon was always successfully removed uninflated. Nevertheless, if one eventually encounters difficulty in removing the balloon, we recommend not using force to avoid balloon damage, but to dilate the balloon in order to allow easier removal after deflation. To facilitate this rescue manoeuvre of disengaging the jailed balloon with a single inflation, we recommend a SB balloon length which is sufficient to exceed the proximal edge of the MV stent.
Regarding the issue of stent distortion, it represents an anticipated drawback of the technique. Accordingly, post-dilation of the proximal part of the MV stent and final kissing inflation are recommended to ensure appropriate apposition of the stent struts to the vessel wall. The bench tests showed that such manoeuvres are theoretically able to completely correct the stent distortion. Yet, in clinical practice, residual proximal stent edge malapposition may occur in the case of incomplete MV stent post-dilation and edge dissection may be caused by incorrect post-dilation of the MV stent inflow. Thus, maximal attention should be paid to the post-dilation phase and, in the case of any doubt of proximal edge dissection or incomplete stent expansion, IVUS evaluation is recommended.
Study limitations
The bench tests have been performed in a single shape of bifurcated phantom with a single type of stent and the intravascular ultrasound evaluation only post-procedurally so that the influence of different bifurcation geometries or stent design on the technique could not be assessed and the stent deformation during the different steps has been explored only by angiography.
The small clinical experience reported does not allow for any evaluation of the technique’s clinical value nor any assessment of the bifurcations subsets (left main, acute angled) more suitable for the technique.
Acknowledgements
Dr. Sianos was the first to use, in clinical practice, a jailed balloon during left main stenting11 and reported his first cases at the European Bifurcation Club Meeting in Prague on September 2008. Drs. Burzotta and Trani were the first to perform bench testing and to apply the technique as a part of a systematic provisional stenting strategy as presented in the present paper. Drs. Burzotta and Trani successfully used the presented technique in a live case transmission at the New Frontiers in Interventional Cardiology meeting (held in Krakow on December 5th 2008).

