
CASE SUMMARY
BACKGROUND: A 62-year-old male was admitted with an acute non-ST-elevation myocardial infarction. Coronary angiography revealed a severe left anterior descending (LAD)-first diagonal (D1) bifurcation lesion, Medina type 1,1,1. Percutaneous revascularisation was performed, using a mini-crush bifurcation technique, with a good angiographic result. Optical coherence tomography (OCT) control was undertaken.
INVESTIGATION: Coronary angiography, OCT.
DIAGNOSIS: D1 stent deformation with severe malapposition caused by a post-dilating balloon, over a guidewire with a course partly outside the stent, resulting in a gap of scaffolding and drug delivery, as well as in a large edge dissection.
MANAGEMENT: Implantation of an additional stent on the proximal D1, by an internal mini-crush technique, sealing the dissection and properly scaffolding the arterial wall, but resulting in an asymmetrical double lumen, separated by a stent neocarina.
KEYWORDS: complication, coronary bifurcation, edge dissection, malapposition, mini-crush technique, neocarina
PRESENTATION OF THE CASE
A 62-year-old, hypertensive, non-diabetic male was admitted to our institution with an acute non-ST-elevation myocardial infarction. Electrocardiography and echocardiography showed normal patterns, without wall motion abnormalities and an ejection fraction of 55%.
Coronary angiography, performed by the transradial approach, revealed a 90% lesion of the left anterior descending (LAD), first diagonal (D1) bifurcation, a second significant stenosis on the proximal D1 and a non-significant lesion on the mid-LAD (Figure 1).
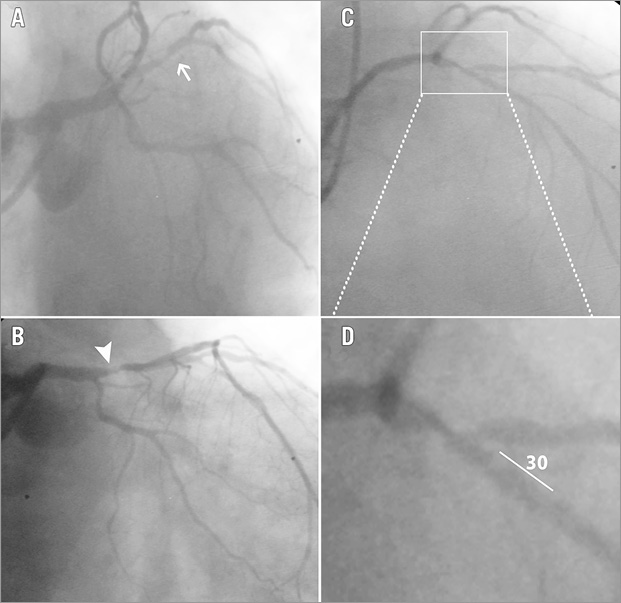
Figure 1. Coronary angiography. Left caudal view showing proximal D1 lesion - arrow (A), right caudal view revealing proximal LAD lesion - arrowhead (B), right cranial view showing the LAD-D1 bifurcation lesion (C), magnification of the white box from panel C, showing the geometry of the bifurcation (D).
The bifurcation lesion was a Medina type 1,1,1, with an acute angle of 30 degrees between distal main branch (DM) and side branch (SB) (Figure 1). A SYNTAX score of 13 was calculated. Therefore, an interventional revascularisation strategy was decided on. Percutaneous coronary intervention (PCI) was performed by the transradial approach, using an EBU 4.0, 7 Fr Launcher® guiding catheter (Medtronic Vascular, Santa Rosa, CA, USA). Due to the significantly diseased ostial and proximal D1, a two-stent bifurcation technique was planned.
A mini-crush technique was performed using two XIENCE Prime™ everolimus-eluting stents (EES) (Abbott Vascular, Santa Clara, CA, USA).
Both LAD and D1 were wired with BMW guidewires (Abbott Vascular), and alternately predilated with a 2.5×15 mm Quantum Maverick™ balloon (Boston Scientific, Marlborough, MA, USA). Subsequently, a 3.0×15 mm balloon was positioned inside the LAD, across the D1 emergence, and a 2.75×18 mm EES was positioned within the D1 and protruded into the LAD with the proximal marker in contact with the LAD balloon. Following deployment of the D1 stent at 14 atm, we crushed it with the 3.0×15 mm LAD balloon. A second 3.0×23 mm EES was deployed in the LAD, across the D1 emergence. The stent diameter was adapted to the DM branch diameter, also taking into consideration the presence of an additional non-significant lesion at this site. The proximal optimisation technique (POT) was performed in the LAD stent with a non-compliant 3.5×8 mm Quantum balloon (Boston Scientific), adapted to the proximal LAD diameter. Subsequently, we failed to rewire the D1 through the side of the LAD stent both with the LAD wire and with a new BMW wire. Therefore, we used a hydrophilic PT2™ wire (Boston Scientific), that re-entered the D1 through the proximal side of the ostium. First, it was impossible to advance a 1.5 mm balloon inside the SB; therefore, we repeated the rewiring process and subsequently succeeded in crossing into the D1 with the 1.5 mm balloon. Finally, a kissing balloon was performed using a 3.5×15 mm balloon into the LAD and a 3.0×15 mm in the D1, and thereafter POT with a 4.0×8 mm non-compliant balloon at 14 atm was carried out inside the LAD stent.
A good angiographic result was achieved (Figure 2A). However, because of the difficulties encountered in wiring the D1, an optical coherence tomography (OCT) (ILUMIEN™; St. Jude Medical, St. Paul, MN, USA) control was performed. Good stent apposition and expansion was documented for the LAD stent. Nevertheless, OCT confirmed our suppositions regarding a possible complication occurring during the SB wiring process. The proximal part of the D1 stent was deformed onto the carina wall, eccentrically, in a semilunar shape, leaving the lateral wall uncovered by the stent, for a distance of 4.3 mm (Figure 2B, Figure 2Ba). The maximal malapposition was 2,100 µm. Moreover, the stentless lateral wall was complicated by a large edge dissection with a width of 890 µm (Figure 2Bb). The distal segment of the D1 stent was normally apposed (Figure 2Bc).
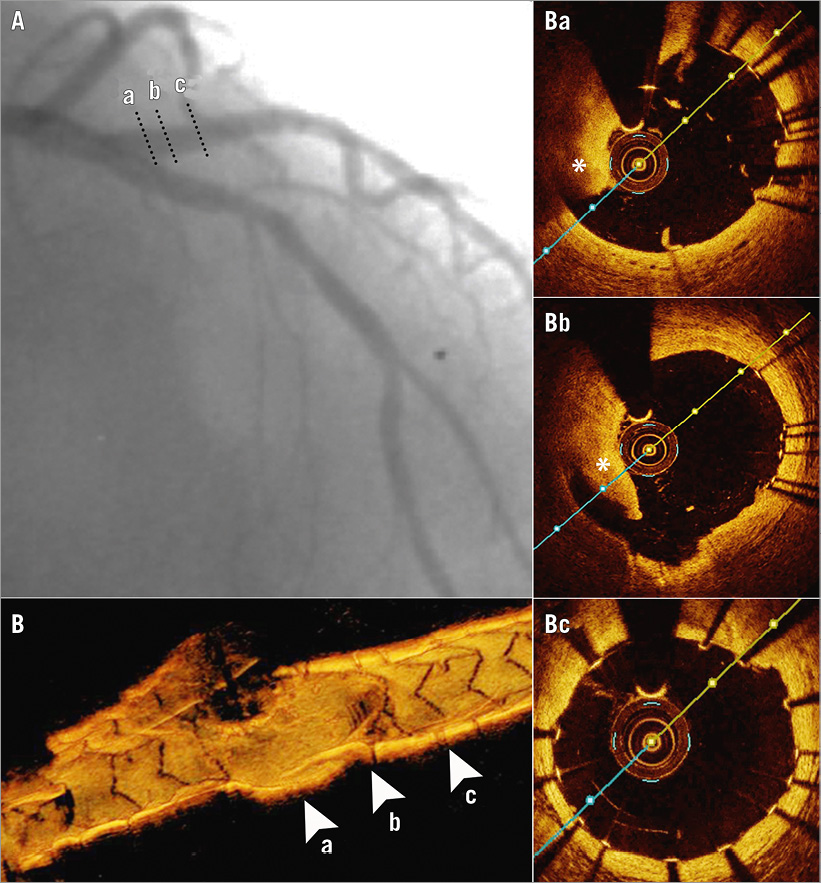
Figure 2. Result after performing mini-crush stenting. Angiogram (A), 3D-OCT of the SB, revealing a stent-free proximal segment (B) and OCT cross-sections on SB, showing proximally the carina wall with the stent struts deformed in a semilunar shape (Ba) and the lateral wall with a large rim of dissection (asterisk) located in the gap of strut scaffolding (Bb). Distal half of the stent is apposed to the wall normally (Bc). Cross-sectional levels are highlighted by dotted lines on the angiogram and by arrowheads on the 3D-OCT reconstruction.
How would I treat?
THE INVITED EXPERTS’ OPINION

Crush stenting is a double stenting technique developed by Colombo et al1 with the aim of assuring full coverage of a bifurcation lesion with stent struts. After more than a decade of practice with the crush technique, we know that it may be performed using various sequences of technical steps and that the performance of a final kissing inflation is pivotal for clinical outcome.
In the reported case, the authors encountered difficulties during side branch (SB) rewiring and noticed (after kissing ballooning) major lack of coverage of the SB proximal segment. Can we explain such a failure and how should we manage it?
The explanation for this situation can be found in bench test experiences. In particular, Ormiston et al extensively investigated the drawbacks of crush stenting2 and noticed that, if the rewiring wire goes, by chance, too proximal/lateral at the SB ostium level, it may pass between the SB stent struts and the SB proximal wall. When this occurs, kissing ballooning is associated with focal removal of stent struts from the vessel wall which translates into proximal SB “uncoverage” by the stent. Was this the problem in this case? The probability is high since the SB was finally accessed with a hydrophilic wire and small balloon, a combination that may be associated with incorrect tracking. Thus, the case shows some important drawbacks of mini-crush stenting in practice and calls for the selection of a more updated technical sequence. In particular, double kissing crush stenting (DK crush)3 may be valuable since, by adding the step of a first kissing ballooning before stent implantation in the main vessel, it has the potential to reduce trouble in final SB rewiring and kissing.
Of note, it should be underlined that invasive imaging (OCT) was used quite late during the reported case since probably, if performed after rewiring, it would have been able to disclose incorrect wire track4, prompting repeat rewiring instead of undersized balloon selection.
Finally, how should we treat? In this regard, we have to recognise that the angiographic result looked fine and that the dissection was recognised only by OCT. So far, the need to react actively to an OCT-detected complication is a matter of discussion. Furthermore, we previously reported that OCT often detects dissections at the SB ostium after bifurcation PCI, even when the simpler technique of provisional stenting is applied5. If action is considered necessary, the selection of the T and protrusion (TAP) technique looks appealing since it is associated with complete ostium coverage and the ability to perform final kissing inflation6. In keeping with this, TAP has recently been highlighted as a possible way to fix SB ostium unintentional “uncoverage”6.
Conflict of interest statement
F. Burzotta has been involved in advisory board meetings for Medtronic and has received speaker’s fees from Medtronic, St. Jude and Abiomed. C. Trani has received speaker’s fees from Abbott, Terumo, Biotronik.
How would I treat?
THE INVITED EXPERT’S OPINION

This is a very educational case which contains some good teaching points. The patient presented with an acute coronary syndrome and a bifurcation lesion, which is always a challenging case scenario. Using a 7 Fr catheter in an acute coronary syndrome case, as well as in complex PCI cases using imaging and plaque preparation tools at the same time, is a very good choice, especially if you are familiar with true radial access. There are many reasons why a 7 Fr catheter could help to finish this case successfully – offering good catheter support, it allows the two-stent technique, etc., to be used.
Another option would be to treat this lesion using the one-stent technique. Figure 1D shows that we could probably manage this case with one stent in the mid and proximal LAD, finishing with “POT+SB-POT” at bifurcation level. We need to remember that we should try to stay simple in acute coronary syndrome cases. On the other hand, the diagonal branch is large and an acute angle of 30 degrees is an indication for choosing a two-stent strategy.
Rewiring the SB strut at the proximal side and performing kissing ballooning after both stent implantations caused SB stent deformation. Probably the DK crush technique would be better than the step-crush technique. In any case, crossing two stent layers is challenging: the right way to start is with a soft wire, as was carried out in this case. It is more difficult because of SB stent deformation and, to fix this complication, you will need to re-cross all the stent strut layers. A better option would be to try to re-cross the SB stent real lumen, using OCT guidance. A 7 Fr catheter allows you to use several devices at the same time and, with a hydrophilic wire, you can try to re-cross the real lumen of the SB stent. If successful, you need to perform a separate post-dilatation with an NC balloon in the SB and MB, finishing with kissing inflation and POT. If not, in worse case scenarios, you will need to perform another post-dilatation to crush the stent completely to the carina wall in the SB. Another kissing and POT should be performed, leaving the dissection to heal with time. The patient should be discharged on triple therapy.
In conclusion, for this patient and even more so for the operator’s learning curve, it is very important to control the result with imaging techniques, especially in two-stent cases with problematic rewiring. Using a 7 Fr catheter it is possible to manage the complication using OCT and the two-wire technique at the same time. In case of failure with rewiring, you need to prescribe patient triple therapy to avoid the occurrence of later events and perform angiography control in six to 12 months.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
This type of complication is prone to two potential hazards: stent thrombosis due to the free stent layer and the large edge dissection inside the SB7,8 and restenosis because of the gap in drug delivery9. In our case, stent thrombosis would have very severe consequences due to the large D1 and the location of the bifurcation, close to the LAD ostium.
Therefore, in treating the complication, we had two goals:
1. To completely crush the deformed stent to the wall with the aid of an additional larger stent in order to achieve a good apposition of the stent layers and decrease the risk of stent thrombosis.
2. To cover the lateral wall and the large edge dissection with an additional stent, reducing the risk of both restenosis and thrombosis.
Having determined the real size of the SB by OCT, we implanted a 3.5×8 mm EES at the D1 ostium, using an internal mini-crush technique. The stent was deployed at 14 atm, with a short protrusion into the LAD which was crushed by a 3.5×15 mm balloon. Kissing was performed with two 3.5×15 mm balloons at 14 atm each. Finally, we performed proximal optimisation with a 4.0×8 mm non-compliant balloon at 16 atm.
The angiographic result was excellent (Figure 3A). A new OCT control (Figure 3B, Figure 3Ba-c) revealed the persistence of the semilunarly deformed stent inside the proximal D1, though greatly reduced in surface, covered by the new 3.5 mm stent (Figure 3Bb). Distally the two stents were concentrically apposed (Figure 3Bc). Additionally, the SB ostium was not entirely cleared of the crushed stent struts, even after the 3.5 mm balloon inflation at high pressures (Figure 3Ba).
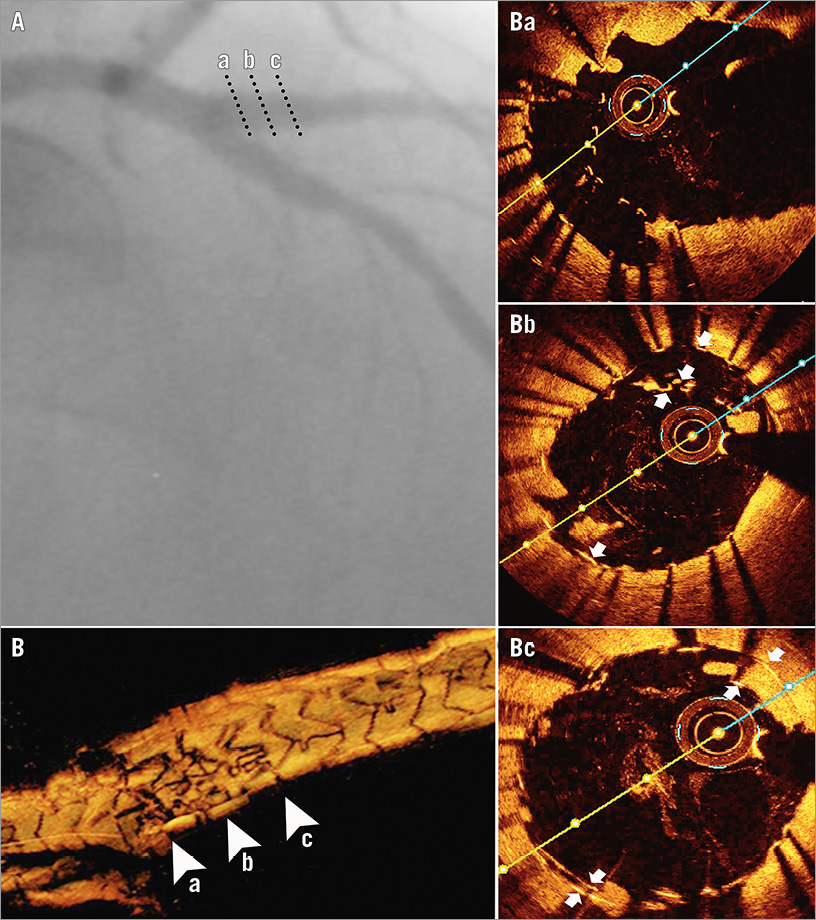
Figure 3. Result on the side branch after the correction strategy. Angiogram (A); 3D-OCT (B) and 2D-OCT cross-sections (arrowheads on 3D-OCT) after deployment of an additional EES by an internal mini-crush technique, showing ostial free stent struts not cleared by final kissing balloon (Ba), proximal asymmetrical double-barrelled appearance of the stents (Bb) and a distal concentric disposal (Bc). Stent layers are highlighted by white arrows.
The patient was left on dual antiplatelet therapy with aspirin and ticagrelor and he remained asymptomatic.
Control coronary angiography and OCT were performed after six months (Figure 4), revealing a good endothelialisation both of the semilunar neocarina and of the struts left in the SB ostium.
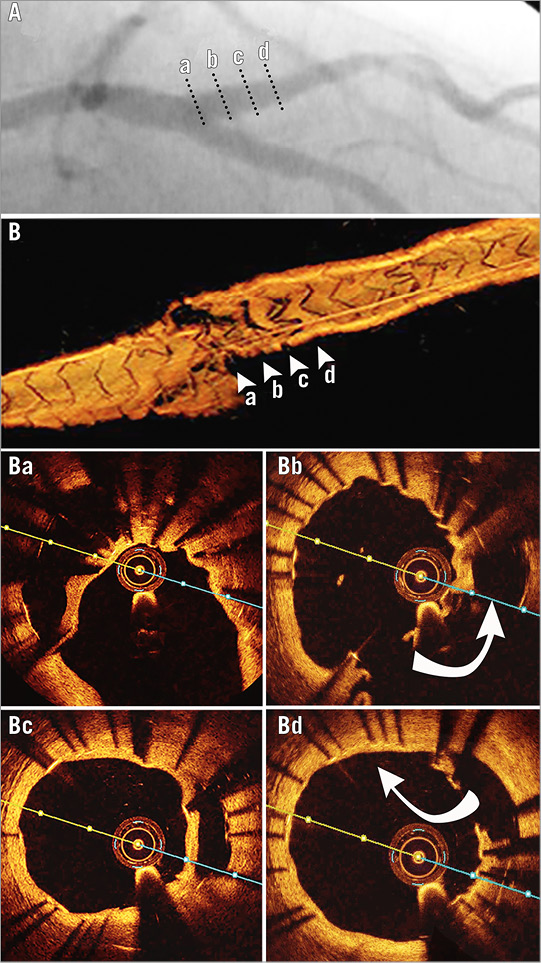
Figure 4. Six-month control on the side branch. Angiography (A), 3D-OCT (B), OCT cross-sections (marked on 3D-OCT by arrowheads and on angiogram by dotted lines): ostial – endothelialisation of the not cleared stent struts (Ba), proximal - endothelialisation of the neocarina (Bc). Entry (Bb) and exit sites (Bd) into the false lumen are highlighted by curved arrows.
Discussion
Recent studies have shown comparable results between one and two stenting bifurcation techniques with second-generation DES10. Because of the diseased ostial and proximal SB, we favoured complete coverage of the SB ostium using a two-stent technique.
We supposed that the mechanism of the aforementioned complication is similar to the one described by Ormiston et al in bench testing with micro-CT visualisation11. It is caused by the post-dilating balloon following an SB wire that had exited the MB and re-entered the SB after a course outside the stent11.
In our case, the stent was undersized for the D1 ostium and it had a very short protrusion into the LAD (Figure 5-1), probably due also to the sharp angle between the two branches. Therefore, crushing it by the LAD balloon (Figure 5-2) yielded a longitudinal deformation by squeezing together the proximal stent struts (“concertina” effect)12. Additionally, the stent struts were probably also projected into the vessel lumen, separating them from the wall as already described in bench testing12. Therefore, whilst rewiring the SB, the guidewire passed underneath the proximal struts and re-entered the stent in its middle part (Figure 5-2). Furthermore, balloon dilatation deformed the proximal part of the stent to the carina wall (Figure 5-3, Figure 5-4). These events yielded a gap in strut scaffolding and drug delivery and also a large edge dissection.
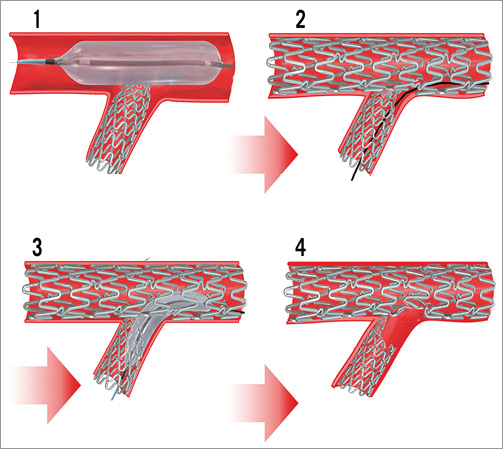
Figure 5. Modelling the stenting complication mechanism. 1) Main branch (MB) balloon, crushing the short protrusion of the side branch (SB) stent. 2) The guidewire used in SB rewiring has passed underneath the deformed proximal SB stent struts. 3) Balloon dilatation outside the SB proximal struts. 4) Large deformation of the SB stent against the carina, creating a gap in stent scaffolding..
In order to meet the potential hazards of stent thrombosis and restenosis, we tried to correct the complication by covering the lateral wall with a second stent (Figure 6). According to OCT studies7,8, the risk of stent thrombosis is not well established for malapposed stents, though such a severe malapposition as ours has not been thoroughly investigated. Nevertheless, in our case the risk of stent thrombosis was further increased by the large edge dissection.
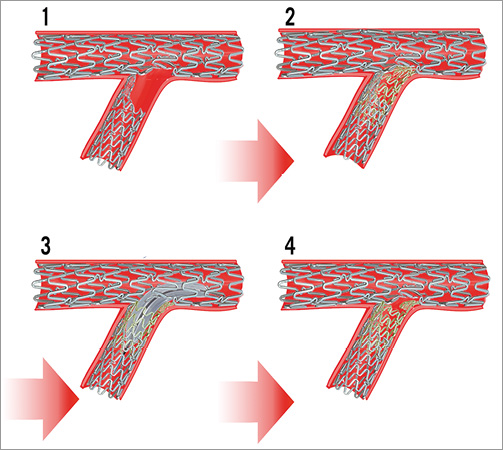
Figure 6. Modelling of the correction attempt. Deploying an additional stent (yellow) by an internal mini-crush technique. 1) Result of the initial mini-crush technique, with proximal SB partially not covered by the stent struts. 2) The gap in the stent scaffolding is covered by an additional stent implantation. 3) Opening of the crushed struts over the SB ostium. 4) Final result.
Unfortunately, our correcting stenting strategy yielded two situations of free stent struts not attached to the vessel wall: first, the neocarina resulted from the kissing of the first deformed stent with the correction stent and, second, the free struts from the SB ostium did not clear during the final internal mini-crush procedure. Theoretically, neointimal coverage on free stent struts might not be expected. However, at six-month control they were entirely covered by neointima (Figure 4). Data about endothelialisation of stent struts not intrinsically attached to the vessel wall are provided from case reports on simultaneous kissing stents13 and studies on stents crossing side branch vessels14. Endothelial healing of DES depends on drug release kinetics and also on polymer type. The XIENCE Prime EES is a second-generation Co-Cr DES with very thin struts, a fluoride copolymer, and a 90% elution of the drug within the first 90 days15, features that could explain the good endothelialisation at six months.
The bench data together with our findings may suggest that this type of complication may not be only one single, incidental episode. Whenever important difficulties are encountered in rewiring and passing a balloon into the SB during a two-stenting bifurcation technique, an OCT control should be undertaken. However, if such a complication occurs, in our opinion, the first objective should be to prevent stent thrombosis and restenosis by a correction strategy together with aggressive antiplatelet medication. Our experience has shown that, in time, good endothelialisation occurs, even on stent struts without a neighbouring intact vessel.
Conflict of interest statement
The authors have no conflicts of interest to declare.

