Abstract
Aims: The aim of this study was to assess the ability of optical coherence tomography (OCT) to guide recrossing during percutaneous coronary interventions in bifurcations and to reduce strut malapposition.
Methods and results: Fifty-two patients undergoing elective treatment of bifurcation lesions using provisional stenting as default strategy were included in the study. Patients were divided into two groups: OCT-guided stent recrossing (group 1, n=12), and angiography-guided recrossing (group 2, n=40). Malapposition in the various bifurcation segments was compared in the two groups, using propensity score analysis to correct for confounders. In 4/12 patients (33%) of the OCT-guided group after the first attempt to recross the stent towards the SB the wire was found to have crossed in a proximal cell, requiring a second and in one case a third attempt to successfully cross through a distal cell. Patients who were treated using OCT-guided recrossing had a significantly lower number of malapposed stent struts, especially in the quadrants towards the SB ostium (9.5%[7.5-17.4%] vs 42.3%[31.2-54.7%] in the angiography-guided group, p<0.0001).
Conclusions: The rate of strut malapposition was significantly reduced when OCT was used to confirm that wire recrossing was performed in a distal cell of the SB ostium.
Abbreviations
CT: computed tomography
DES: drug-eluting stent
FD-OCT: frequency-domain optical coherence tomography
IVUS: intra-vascular ultrasound
LAD: left anterior descending artery
LCx: left circumflex artery
LM: left main
MV: main vessel
MLA: minimum luminal area
OM: obtuse marginal artery
PCI: percutaneous coronary interventions
RCA: right coronary artery
SB: side branch
SKS: simultaneous kissing stents
TAP: T and protrusion technique
Introduction
Provisional stenting is the generally accepted technique for treatment of bifurcations, with the need of a second stent in the side branch in 5-30% of true bifurcations.1-12 With the disappearance of the crush technique and the use of SKS stenting only for niche indications, a distal position of wire recrossing is equally important irrespective of whether a single stent is used or a second stent (T-stenting/TAP or culotte techniques) is required because of dissection and slow flow in the side branch.1,6,13-15 Angiography and IVUS are unreliable at detecting the site of wire recrossing. The superior resolution and rapid acquisition of FD-OCT makes this technique ideal to locate the position where the guidewire recrosses the stent struts towards the side branch. We performed serial OCT examinations to ensure recrossing via a distal cell. In a consecutive series of bifurcation procedures we compared strut malapposition after FD-OCT guidance with the results from historical controls.2,5,6,16
Methods
PATIENTS
The study population consisted of 52 patients undergoing elective bifurcation lesion stenting. Patients were divided into a group with OCT-guided wire recrossing (n=12), and a group with angiography-guided recrossing with final OCT assessment (n=40).
The main exclusion criteria were the presence of a side branch smaller than 2 mm in diameter (visual estimation), ST-segment elevation acute myocardial infarction (<48 hours), severe heart failure (NYHA Class >3) and renal insufficiency (creatinine clearance <60 ml/min or total creatinine >150 mmol/dL).
ANGIOPLASTY PROTOCOL
After loading with clopidogrel (300 or 600 mg) or prasugrel 60 mg and aspirin 300 mg, patients received bivalirudin or unfractionated heparin (to maintain ACT>250 sec checked every 30-60 min). Procedures were performed using radial or femoral access and 6 or 7 Fr guiding catheters at the operator’s discretion. We used a primary provisional stenting strategy with final kissing balloon dilatation with implantation of a second stent only in the event of side branch compromise. Predilatation was performed in all patients for the MV while the SB was dilated before stenting only if there was flow compromise after MV predilatation. We always maintained a jailed wire in the SB after the procedure to straighten the SB origin and facilitate the identification of the SB ostium. A third wire was advanced into the distal MV with a small loop in an attempt to recross the stent struts distal to the jailed SB wire. When a second stent was required, either the T-stent/TAP or the culotte techniques were used with selection based on the relative diameter of MV and SB (T-stent/TAP preferred for SB smaller than MV) and the angle between the daughter vessels (culotte preferred for swallow angles).
In group 1, OCT was performed after wire crossing. If the position of the wire was not located in the most distal cell, further attempts to redirect the wire to a more distal cell were performed, with subsequent OCT acquisitions to confirm position, both in 2D and on-line by 3D rapid reconstruction available in the OCT software. Both groups received kissing balloon dilatation using balloons matching the size of the side branch and of the distal MV. If a second stent was inserted, the procedure was followed as above for T-stenting (or TAP), while for culotte a further OCT acquisition was performed to guide wire recrossing through the distal cell. In group 2, wire recrossing into the SB was performed using conventional angiographic guidance. Details of the PCI procedures including balloon diameters, stent type and size were recorded. Stent optimisation and final kissing dilatations were performed with non-compliant balloons. Both groups received a final OCT assessment after proximal optimisation and kissing balloon dilatation.
FD-OCT IMAGE ACQUISITION AND ANALYSIS
FD-OCT was performed using a C7 system (LightLabs®; St Jude, Minneapolis, MN, USA) and a DragonFly imaging catheter. Automatic pullbacks were performed at 20 mm/sec during contrast injection at a rate of 3-5 ml/sec using a power injector. The DragonFly catheter was inserted distal to the MV stent and the pullback continued until either the guiding catheter was reached or the maximal pullback length (5.5 cm) was completed. This ensured adequate imaging of the entire MV stent. OCT examination was performed after SB wire crossing and, if required, repeated after wire repositioning in group 1 (Figure 1). A final OCT pullback was performed after proximal optimisation and kissing balloon dilatations in all groups.
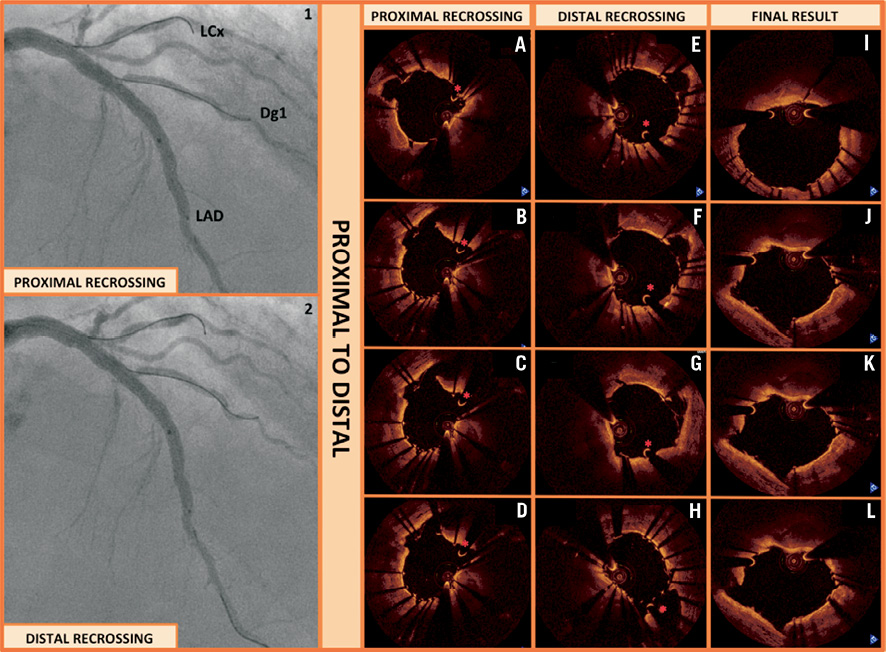
Figure 1. OCT assessment of stent recrossing. An 81-year-old male with LAD-first diagonal branch (Dg1) lesion. A provisional stenting strategy was followed. After predilatation with 2.5 and 3.0 balloons, a 3.0×38 mm Xience Prime stent (Abbott Vascular) was implanted and post-dilated with a 3.5 non-compliant balloon. Proximally, we implanted a second Xience Prime stent (3.5×23 mm) that was post-dilated to 4.0 mm. A new guidewire was inserted into the diagonal branch. OCT revealed a very proximal crossing of the wire (Panel 1, A-D). Thus, a second wire was inserted into the diagonal branch through a more distal stent cell, confirmed by a new OCT pullback (Panel 2, E-H). Angiographically (Panels 1-2), the position of the two wires crossing into the diagonal branch looked very similar, and only OCT was able to detect the difference. Final kissing balloon inflation was performed showing good expansion of the stents and apposition in the critical segments represented by the ostium of the diagonal branch (Panel 3, I-L) (MLA of 9.03 mm2 and MLD of 3.11 mm).
Quantitative, off-line analysis of the OCT images was performed using proprietary computer software (LightLab Imaging, St Jude, Minneapolis, MN, USA). Frame by frame cross-sectional images were analysed by an experienced operator, counting every single strut on each frame. Minimum areas and diameters of both the lumen and stent as well as strut malapposition were measured.
Incomplete stent apposition was defined as separation of at least one stent strut from the vessel wall. A strut was considered incompletely apposed if the distance from the endoluminal surface of the strut to the vessel wall was higher than the sum of the metal and polymer thickness. The cutoff points used for each stent type were: paclitaxel-eluting stent (TAXUS, Boston Scientific Corp., Natick, MA, USA) 130 µm, sirolimus-eluting stent (Cypher Select, Cordis, Johnson & Johnson, Miami, FL, USA) 160 µm, everolimus-eluting stent (Xience V, Abbot Vascular, Santa Clara, CA, USA) 90 µm, ≥110 μm for zotarolimus-eluting stents (Resolute Integrity, Medtronic, Minneapolis, MN, USA) and biolimus-eluting stent (Biomatrix III, Biosensors, Morges, Switzerland) 120 µm. The number of malapposed struts was measured.
STATISTICAL ANALYSIS
Statistical calculations were performed using R version 2.13.1. Numerical values are presented as mean and standard deviation or median [interquartile range] as appropriate. Comparisons between groups were performed using Wilcoxon rank sum test. Propensity scores were used to create a covariate that summarises confounders within baseline demographic and angiographic characteristics and assesses the independent effect of treatment technique on malapposition, defined as percentage of malapposed struts. In short, a logistic regression model was constructed predicting the probability that a patient with certain characteristics would be treated by standard or OCT-guided technique.17 Independent variables in the logistic regression model included baseline clinical and angiographic parameters: sex, age, diabetes mellitus, presence of a true bifurcation, acute coronary syndrome and type of stent used. The C-statistic of the model was 0.87, suggesting very good accuracy in predicting the type of treatment (OCT guided wire recrossing–group 1- vs. angio-guided recrossing –group 2). All patients received an estimated propensity score and were divided into quartiles of propensity score. To estimate the impact of treatment technique on malapposition, a linear regression model was used with percentage malapposition as dependent variable and treatment type as well as quartiles of propensity score as independent variables. Given the small sample size, the confidence intervals (CI) for the average difference in % of malapposed struts between patients in the two groups were derived by bootstrapping (R package boot, 10,000 replications) to improve accuracy. A p value of <0.05 was considered indicative of statistical significance.
Results
PATIENT CHARACTERISTICS
Patient characteristics are summarised in Table 1. Age, sex, smoking history, hypertension, hypercholesterolaemia, diabetes and a family history of ischaemic heart disease were equally distributed in both groups.
The majority of the patients presented with stable angina and less than 10% were admitted with a non-ST segment elevation acute coronary syndrome. Of the patients in both groups, 75% had documented inducible ischaemia and more than 66% of the patients in both groups had had a previous MI, PCI or CABG. Average left ventricular ejection fraction was preserved in both groups.
There was a significantly higher prevalence of right coronary artery bifurcation treatment in group 1 compared to group 2. The frequency of Medina “true” bifurcations (1,1,1; 0,1,1; 1,0,1) was equal in both groups.
Vessel characteristics were similar in both groups, with a mean of 2.8 and 2.9 mm main vessel distal reference diameters measured by OCT (groups 1 and 2, respectively).
STENT MALAPPOSITION
Procedural characteristics and percentage of malapposed struts are summarised in Table 2 and Table 3. Patients who were treated using OCT guidance had a significantly lower number of malapposed stent struts (median 7.5% [5.8-8.8%]) compared to those undergoing stenting with angiography guidance (15.8% [11.3-24.3%], p=0.0009). The significant difference in apposition rate was maintained even after adjustment for confounders using propensity score quartiles: average 11.4% fewer malapposed struts, 95% CI: 5.3-22.6%). OCT guided patients were also less likely to have more than 10% malapposed stent struts, even after adjustment for confounders (OR 0.08, 95% CI: 0.01-0.81). A significant difference was also observed when distal and proximal segments of the stent were analysed (Table 3, Figure 2).
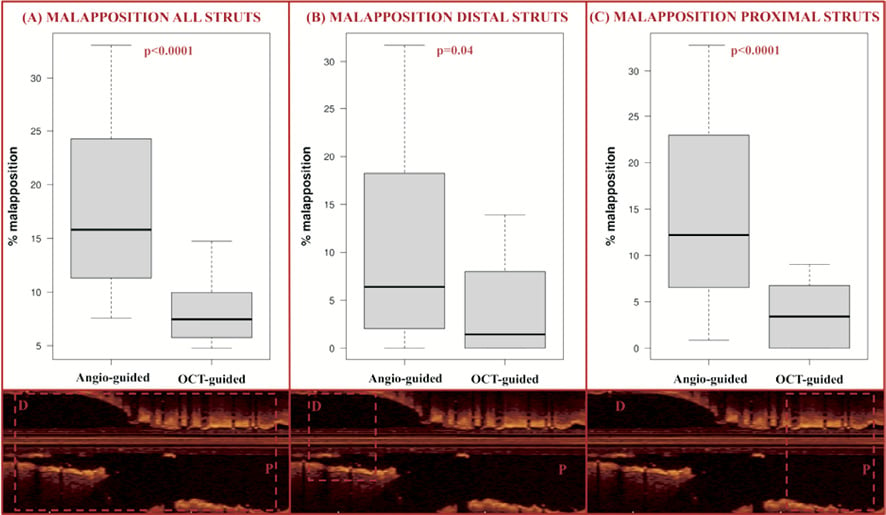
Figure 2. Strut malapposition. The percentage of malapposed struts was measured by OCT. After kissing balloon inflation, the rate of malapposed struts was significantly lower in the OCT-guided wire recrossing group compared to the angiography-guided group, when assessing all stent struts (A), the struts distal to the bifurcation (B) and the struts proximal to the bifurcation (C), as illustrated by the OCT images (inferior panels).
In the bifurcation site, patients who were treated using OCT guidance had a significantly lower number of malapposed stent struts towards the SB (median 9.5% [7.5-17.4%]) compared to the patients with angiography-guided treatment (42.3% [31.2-54.7%], p<0.0001). The significant difference in apposition rate was maintained even after adjustment for confounders using propensity score quartiles: average 26.5% fewer malapposed struts, 95% CI: 15.1-36.9%). A significant difference was also observed when analysing the whole bifurcation segment and the site opposite to the SB (Table 3, Figure 3).
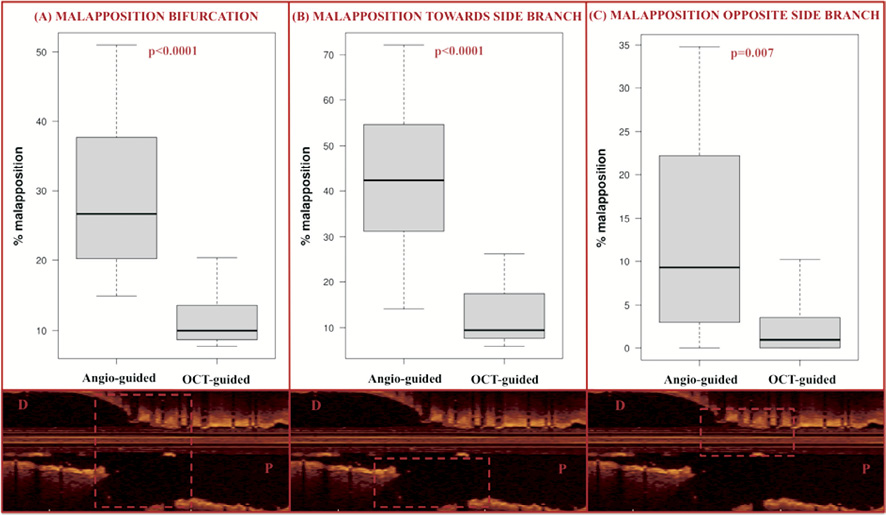
Figure 3. Strut malapposition at the site of bifurcation. The rate of malapposed struts was significantly lower in the OCT-guided group compared to the angiography-guided group when we analysed the struts in the bifurcation (A), the struts towards the side branch opening (B) and the struts opposite to the SB opening (C), as illustrated by the OCT images (inferior panels).
SAFETY AND FEASIBILITY
Due to the high pullback speed, the average amount of contrast required during OCT pullbacks was 17.1±4.2 ml (group 1) and 18.5±3.9 ml (group 2) (p=0.54). None of the complications reported for the previous slow acquisition time-domain OCT (chest pain, ventricular tachyarrhythmia, heart block, severe bradycardia) and no OCT catheter induced coronary dissection or embolism were observed. The mean time added to the procedure by the serial OCT examinations was 20.1±9.4 and 16.2±8.1 minutes (groups 1 and 2, respectively, p=0.13), including OCT catheter preparation, connection to the power injector, activation of OCT system and insertion/withdrawal of the OCT catheter. The average total amount of additional contrast per patient required for the serial OCT examinations was 70.8±19.3 ml (group 1) and 63.3±15.4 (group 2) (p=0.22). The mean creatinine value after the procedure was 85.7±14.3 and 87.7±16.2mmol/l respectively (group 1), and 98.8±23.5 and 96.2 (22.7) mmol/l (group 2) (p=0.14).
No contrast-induced nephropathy was observed. Increase of TnI more than 3-fold baseline value was observed in 33.4 and 42.5% (p=0.056) of the patients in group 1 and group 2, respectively.
Discussion
Optical coherence tomography had always been considered a research tool with no role in the practical guidance of interventional procedures because image acquisition was cumbersome requiring blood displacement. The rapid pullback allowed by the development of frequency domain OCT has simplified the procedure, allowing repeated passes during interventions.18,19 The additional contrast required in the OCT guided group was greater but not significantly different from the control group probably only because the pull-backs were more focused on the bifurcation of interest. Still 70.8 ml of contrast and a maximum number of four pull-backs required only in 1/12 cases appear tolerable for most patients. The superior resolution of OCT offers the advantage of a precise assessment of strut apposition. Previous data from our centre and others detected the presence of 5-10% malapposed struts in complex lesions20,21, but a staggering 42.9% malapposition in the bifurcation sub-segment where the struts face the side branch ostium22,23. In straight segments, larger balloons matching the lumen diameter may help to reduce malapposition and we observed a similar trend in our initial experience when the rapid pullback of frequency domain OCT allowed multiple pullbacks across the stented segment.24 This may account for the reduction in malapposition observed in the OCT-guided group proximal and distal to the bifurcation. In bifurcation lesions, however, an OCT after optimisation of treatment with kissing balloons has limited possibilities to lead to further steps toward optimisation. The strategy applied in this study stems from these early disappointing OCT findings and applies the knowledge derived from in vitro and micro-CT studies to the practical treatment of bifurcations (Figure 4). Crossing the distal cell of the stent facing the SB ostium has been shown to offer an improvement of the final result in bifurcations by displacing one to two rows of struts towards the inner curvature of the SB and generating a metal flap preventing plaque recoil in a segment prone to plaque formation. The carina is almost always disease free and the proximal displacement of all the stent struts proximal to the carina can only be beneficial because it substantially reduces malapposition. Operators already try to achieve this goal during wire recrossing after stent deployment by pushing the guidewire distal in the main vessel and then slowly withdrawing it until it falls into the most distal stent cell opening towards the SB. Unfortunately, as expected, this strategy appears to not be effective in all cases and leads to a result only slightly better than random crossing. This is shown indirectly by the high percentage of malapposition in our historical series of documentary OCT in bifurcations and directly by the results of this study showing a proximal crossing in 33.3% (4/12) cases in the first attempt (Table 3). This study also shows that a correct distal recrossing is achievable in all the remaining cases, mainly at the second attempt. The application of a consistent strategy prompting the operator to recross more distal reduces absolute malapposition towards the side branch by 32% and relative malapposition by more than 80%.
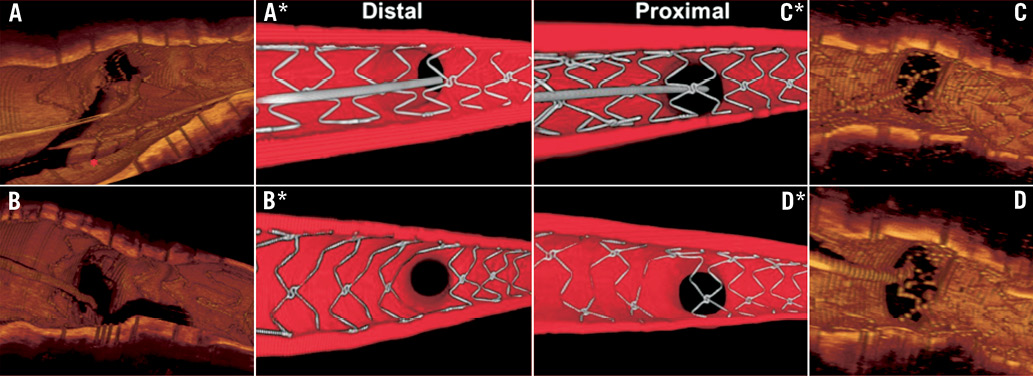
Figure 4. Evaluation of the importance of cell recrossing with 3D OCT and in a representative coronary bifurcation bench model. 3D-OCT. An 81-year-old male with LAD-first diagonal branch (Dg1) lesion after provisional stenting with a 3.0×38 mm Xience Prime stent (Abbott Vascular), post-dilated with a 3.5 mm non-compliant balloon. 3D OCT confirmed the crossing of the guidewire through a distal cell (A). The red asterisk indicates the jailed guidewire in the MV. After KB inflation, we observed a wide opening of the stent into the SB, with no malapposed struts (B). A 72-year-old woman with a severe lesion in the bifurcation of the LAD-Dg1. The 3D OCT image shows the struts of a 3.0×24 mm Integrity RESOLUTE® stent covering the ostium of the side branch. Panel D indicates that despite dilatation of the diagonal ostium with a 2.5 mm balloon at 14 Atm a gross malapposition of the main vessel stent struts persists at the origin of the diagonal. The wire is clearly positioned in the most proximal cell (C). Bench model. Two identical 3 mm coronary stents (BioMatrix Flex, Biosensors) were implanted in the MV of the model with the SB reaccessed either through a distal cell (A*) or the most proximal cell (C*). Results after dilatation of the SB and final KB (using 2.5 mm NC balloon in the SB and 3.0 mm balloon in the MV simultaneously inflated at 10 Atm) show the optimal opening of the strut with distal recrossing (B*) compared to the large residual strut malapposition near the carina with proximal cell recrossing (D*).
We believe that this is the first study strongly suggesting that OCT can be used to improve results of stenting in bifurcation lesions but we are also aware of its many limitations. This is not a randomised trial and the comparison with a historical series may lead to differences in the characteristics of the lesions treated, devices used and technique employed. All patients in both groups received kissing balloon dilatation and proximal optimisation with balloons of similar diameter. Still other differences, for instance in stent type, were present and we agree that even the sophisticated statistical methods we applied to eliminate these confounders have limitations. Therefore, this pilot study should be considered hypothesis generating and requires a multicentre trial to validate this approach.
A more radical criticism may be why we bothered at all with the dilatation of the side branch when no differences in major clinical endpoints were observed in the main trials comparing kissing balloon dilatation with the absence of further procedural steps after the deployment of the stent across the SB origin.25,26 This was difficult to explain knowing that pathology indicates that a high concentration of malapposed struts in consecutive cross-sections is a risk factor for late stent thrombosis27 and bifurcation stenting appear to induce a four-fold increase of the risk of thrombosis irrespective of the technique used for treatment.14 The absence of the expected improvement with KB can be explained by our results, suggesting that if KB dilatation is performed with proximal wire crossing, malapposition is not much better than the malapposition present leaving the side branch ostium jailed with all struts malapposed.
In spite of the consistent achievement of distal cell crossing controlled with OCT, 9.5% of the struts remained malapposed at the SB ostium. Limitations to the deformation of the stent cells, a too small diameter of the balloon used for SB dilatation, unfavourable alignment of the cells forcing a non-central crossing of the wire, may account for the residual malapposition. The clinical relevance of a more modest malapposition in comparison with the 42.3% observed in historical series is unknown. The possibility to assess in vivo with OCT stents of different design and characteristics may favour the development of better stents with a more suitable design for provisional bifurcation stenting.
In addition, new OCT applications as three-dimensional (3D) reconstruction have shown to be potentially useful in this setting and have shown new insights of bifurcational stenting28,29. Okamura et al30 have reported the initial experience using 3D-OCT reconstruction to assess wire re-crossing position during bifurcational stenting.
Conclusion
In this consecutive series of bifurcation lesions strut malapposition was significantly reduced when wire recrossing was performed in a distal cell of the side branch ostium guided by OCT.
Acknowledgements
This project was supported by the NIHR Cardiovascular Biomedical Research Unit of Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. This report is independent research by the National Institute for Health Research Biomedical Research Unit Funding Scheme. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. Dr. Alegria-Barrero received a research grant from the Spanish Society of Cardiology.
Conflict of interest statement
Carlo Di Mario received research grants to the Institution provided by Medtronic (RESOLUTE III trial), Abbott (EXCEL trial), Biosensors (LEADERS allcomers and OCT substudies). St Jude LightLab sponsored live transmissions from the Royal Brompton Hospital. None of the other authors have any conflicts of interest to declare.

