Case summary
Background: A 41-year-old female with 90 minutes of severe chest pain and ST-elevation in leads V1-V6 underwent emergency coronary angiography with a view to primary angioplasty.
Investigations: Physical examination, electrocardiography, coronary angiography
Diagnosis: ST-segment elevation anterior myocardial infarction
Management: Coronary angiography, antiplatelet and anti-thrombotic therapy, statin, angiotensin-converting enzyme inhibitor, beta blocker, IVUS and percutaneous coronary intervention (PCI)
Keywords: acute myocardial infarction, PTCA
How should I treat?
Presentation of the case
A 41-year-old female with 90 minutes of severe chest pain and ST-elevation in leads V1-V6 was transferred directly to our cardiac centre. She was loaded with 600 mg of aspirin and clopidogrel and then underwent emergency coronary angiography, with a view to primary angioplasty. The left coronary artery (Figure 1) showed smooth narrowing of the left main (LM) and proximal left anterior descending (LAD) coronary artery.
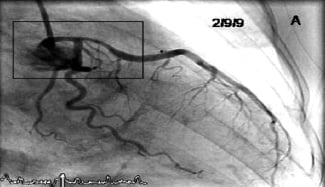
Figure 1. The presentation of acute anterior STEMI with LAD reperfusion.
The RCA was normal. At this time, TIMI III flow was present in the infarct related artery and the chest pain had eased significantly. It was unclear, at this stage, which revascularisation option would provide the best long-term outcome for the patient; therefore we stopped to discuss the options.
How would I treat?
The Invited Experts’ opinion
Dr. Murray et al presented a 41-year-old female with 90 minutes of severe chest pain and ST- elevations in V1-V6. After loading the patient with aspirin and clopidogrel angiography revealed a smooth narrowing of the left main (LM) and proximal anterior descending coronary artery (LAD) with a TIMI III flow and a normal right coronary artery. Before giving my treatment advice, I would first like to know the patient’s cardiovascular history and her risk factors. Since this information was not mentioned in the case presentation my opinion about “How to treat” is based on, hopefully, smart speculation.
Considering the age and sex of the presented patient and the appearance on the angiographic image, it is suspected that we are dealing here with a spontaneous coronary artery dissection (SCAD) in the left main, with a haematoma extending towards the proximal LAD. In the literature, this condition is described as mainly presenting in the younger female population1,2. Recently Shamloo et al published an overview of SCAD aggressive vs. conservative therapy3. Based on 344 cases of SCAD in literature, the authors conclude that early intervention in SCAD patients produces a better outcome and less need for further intervention compared to a conservative approach. However, the limitation of the study is that only 60% of the cases, only those that had a second angiogram, were analysed. No other MACE, apart from re-intervention, was taken into account. Several case reports as well as a series of five patients describe a spontaneous revealing of the dissection4-7. Also complications after stenting SCAD like revascularisation due to the progression of the dissection6 and in-stent restenosis are described8.
Summarising:
Murray et al stopped (temporarily) the procedure to discuss the options:
My options for the presented patient considering the above:
1. To diagnose or rule out SCAD. In this case I would perform IVUS of the LAD and LM, where the left main ostium should be visible, preferably with high frequency, mechanical catheters like the 40 Mhz Atlantis® (Boston Scientific, Natick, MA, USA) or 45 MHz Revolution® (Volcano Rancho Cordova, CA, USA). This will reveal if we are indeed dealing with SCAD and, if so, the length of the haematoma and the origin of the dissection. Be careful not to misinterpret a subintimal or sub medial haematoma for an eccentric fibrous plaque.
2. In case that IVUS reveals a significant stenosis, based on plaque, with the left main stem involved, revascularisation by stenting or CABG by a drug-eluting stent.
3. In case that IVUS confirms SCAD, continue the aspirin, clopidogrel and a beta-blocker and monitor the patient closely for a few days. If the condition of the patient does not improve or if the chest pain re-occurs, the angiogram can be repeated for revascularisation by CABG or stent. The IVUS findings may help to determine the length and size of the stent.
4. If no further complaints occur, it still may be advisable to perform a control angiogram after six months.
How would I treat?
The Invited Experts’ opinion
The goal of primary percutaneous coronary intervention in patients presenting with STEMI is to provide a timely restoration of capillary blood flow to the infracted area in order to preserve myocardial viability. While mechanical intervention is targeted to the epicardial coronary territory at the site of occlusion, interventionalists should not forget that a residual coronary stenosis in the culprit vessel is an acceptable result, provided normal anterograde flow is restored. Indeed, in the presence of a large thrombus burden at the culprit lesion, the trade-off between optimal stent expansion and distal embolisation with subsequent microcirculatory obstruction even in the presence of apparently normal TIMI 3 flow in the epicardial artery is well known. Murray and colleagues have reported in the Journal a very interesting case of a STEMI patient with spontaneously reperfused infarct related artery which poses a frequent dilemma in our practice: Should I just stent this lesion? Or, given the fact that the EKG is normalised and the patient is symptom-free, should I just leave this lesion for the time being?
This question is particularly relevant here for at least three additional considerations: i) the lesion does seem tight at angiography, but its functional significance is unclear based on a single projection, ii) the lesion involves the left main and proximal LAD, a coronary territory with very clear prognostic implications due to the amount of subtended myocardium whose treatment should necessarily imply carefully weighted of pros and cons, iii) the patient is a very young lady in whom the presence of a dynamic stenosis is relatively frequently found. Additionally, the culprit lesion seems to be smooth at angiography, with no clear evidence of plaque rupture nor thrombus based on the still frame provided of the left coronary system.
Therefore, there is no pressure on a timely intervention as myocardial reperfusion has already spontaneously occurred and the decision on how to proceed has to be taken. At this stage, I would refrain from stenting the culprit lesion for the reasons provided above and I would proceed to a left ventricular angiogram to confirm and detect the presence of balloon-like asynergy of the apical region which is a typical feature of the Tako-Tsubo syndrome. The pathophysiology of the syndrome is controversial and several hypothesis are considered, including cathecolamine-mediated myocardial stunning and endothelial dysfunction. Although there are contrasting opinions about the role played by coronary spasm, there is at least one published case in the literature with angiographic documentation that coronary spasm may trigger this condition1.
Intracoronary nitrates and/or verapamil or other calcium channel blockers should be given intracoronary to possibly resolve coronary spasm and intravascular imaging such as intravascular ultrasound and/or optimal coherence tomography should be undertaken to confirm or exclude the presence of atherosclerotic disease.
Provided intracoronary imaging modalities unequivocally confirm the presence of potential flow-limiting atherosclerotic plaques in the proximal LAD and left main, immediate or delayed (i.e., in a staged procedure) left main-LAD cross-over stenting appears a reasonable treatment strategy in a young patients with very low SYNTAX score.
How did I treat?
Actual treatment and management of the case
A conservative medical approach was taken initially, to allow for discussion of the relative advantages and disadvantages of LMS/LAD stenting versus coronary artery bypass surgery. The patient remained on our coronary care unit for seven days treatment with aspirin, clopidogrel, abciximab, enoxaparin, atorvastatin, bisoprolol and ramipril. The case was shown in our multi disciplinary meeting to gauge opinions on the best treatment options. However, after full discussion with the patient, she opted for percutaneous coronary intervention (PCI) and eight days after her initial event, when completely asymptomatic, she returned to the catheter lab. Repeat angiography showed significant improvement in the diameter of both the LM and LAD coronary arteries with no significant stenoses remaining (Figure 2B). However, the magnified view (Figure 2C) showed clear evidence of a “roof ulcer” in the LM that had filled with contrast agent (encircled). Further imaging with intravascular ultrasound (IVUS) shows the grey scale appearance of a fissured plaque proximal to the LM ulcer (Figure 2E) and the virtual histology (VH) appearance of a relatively “high-risk” plaque with significant areas of necrotic core (red) (Figure 2F). Figure 2G depicts the grey-scale appearance of the ulcer communicating with the LM lumen and Figure 2H shows the white speckling of injected contrast medium entering the ulcer. Figure 2I also demonstrates that even after rupture this plaque still contains some high risk features, with necrotic core still classified in the rupture associated plaque (RAP).
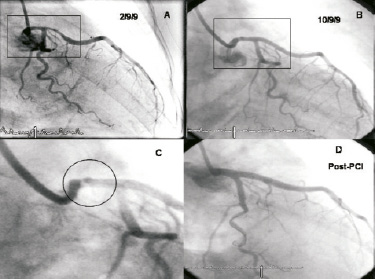
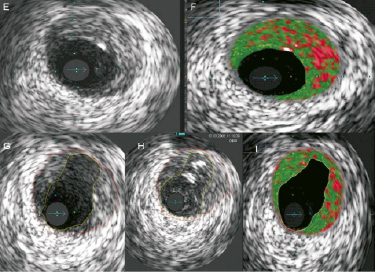
Figure 2. (A-D). (A) is shown again to allow comparison from the initial angiography on presentation and (B) and (C) showing what is possible with aggressive antiplatelet and anti-coagulation for oneweek. (D) is the final result of PCI. (E-F). The intravascular ultrasound and virtual histology (VH) appearance of the plaque present in the left main stem. (E) and (F) show a soft, fissured fibroatheromatous plaque with significant necrotic core present. (G) shows the roof ulcer cavity within the plaque and (H) was taken as contrast was injected into the ulcer. (I) is the virtual histology representation of rupture associated plaque (RAP) which still contains some high-risk features.
Following analysis of the angiographic and IVUS images, it was felt that to treat the residual disease appropriately we should stent from normal to normal and ensure coverage of the left main stem ulcer by stenting back to the ostium. We placed a 3 × 18 mm PROMUS™ stent (Boston Scientific, Natick, MA, USA) in the proximal LAD, overlapped by a 4 × 18 mm PROMUS™ in the left main stem, unfortunately there was significant plaque shift into the circumflex ostium and the vessel closed briefly. This was wired promptly and a 3 × 15 mm PROMUS™ was placed in a T-fashion up to the ostium of the circumflex. The entire stented segment was postdilated sequentially and in a kissing balloon fashion between LMS and circumflex. Figure 2D shows the final angiographic result.
Conclusion
This case highlights well the rare angiographic appearance of plaque rupture/ulceration in the LM coronary artery and illustrates its thrombotic consequences. It also provides support for the commonly seen IVUS finding that the true culprit lesion in any acute coronary syndrome (ACS) may be filled and concealed initially by thrombus, and often lies somewhat proximal to the angiographic minimum lumen diameter (MLD) point. In order to adequately cover ACS-related ruptured plaque and prevent the risk of further thrombotic complications after PCI, intravascular imaging may be a very useful tool after thrombus extraction.