CASE SUMMARY
BACKGROUND: A 45-year-old woman presented to the emergency department with ST-segment elevation myocardial infarction (STEMI).
INVESTIGATION: Physical examination, electrocardiography, coronary angiography, echocardiography, cardiac computed tomography.
DIAGNOSIS: STEMI due to spontaneous left main coronary artery dissection involving left anterior descending, intermediate and left circumflex arteries.
TREATMENT: Percutaneous coronary intervention (PCI).
KEYWORDS: Acute myocardial infarction, left main spontaneous coronary artery dissection, multivessel disease
PRESENTATION OF THE CASE
A 45-year-old female smoker with a family history of coronary artery disease presented to the emergency department (ED) with atypical chest pain lasting for one hour.
She had no past medical history and her only medication was oral contraceptives (gestodene and oestrogen). On arrival at the ED the patient was asymptomatic and haemodynamically stable.
As the patient confessed that she had been suffering from dry cough over the last few days, the first clinical suspicion was pneumonia. A chest radiogram and serological inflammatory blood tests were performed and were within the ranges of normality. Two hours after admission the patient developed vomiting and hypotension. The electrocardiogram showed sinus rhythm, upsloping ST elevation, hyperacute T waves in the precordial leads, and the first troponin I was minimally increased (0.049 mg/L).
The patient was treated with aspirin and prasugrel and was finally transferred to our cathlab for an emergent coronary angiogram. The coronary angiogram showed a long, subocclusive, flow-limiting dissection of the left main (Figure 1A and Moving Image 1). The shaft of the left main clearly showed an intraluminal flap compressing the true lumen; moreover, the angiogram revealed a diffuse reduction of the lumen of the left anterior descending, intermediate and left circumflex arteries with TIMI 1 flow. Conversely, the right coronary artery was normal (Figure 1B).
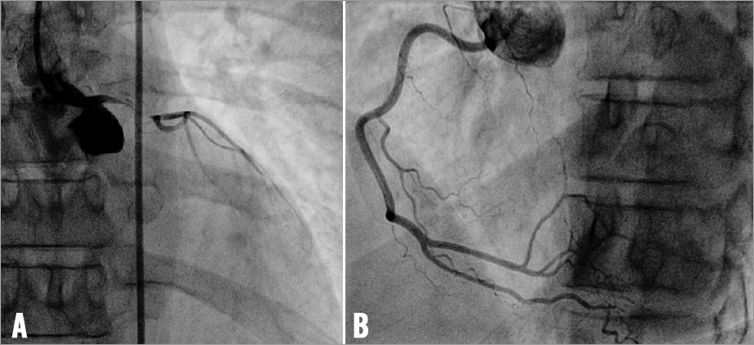
Figure 1. Basal coronary angiography shows a long subocclusive, flow-limiting dissection of the left main and diffuse reduction of the lumen of the left anterior descending, intermediate and left circumflex arteries with TIMI flow grade 1 (A), and normal right coronary artery (B).
How would I treat?
THE INVITED EXPERTS’ OPINION
Spontaneous coronary artery dissection (SCAD)
This case illustrates the classical presentation of patients suffering from spontaneous coronary artery dissection (SCAD) of the left main coronary artery. Performing a Boolean search on PubMed with “(left coronary or left main) and (spontaneous coronary dissection or dissecting coronary aneurysm or primary dissecting aneurysm) and coronary” permits identification of 739 publications on this particular topic. The present case is classical, since the patient is a woman (>70% of the cases published so far), on oral contraceptives (pills or pregnancy is found in most of the female patients), and is 30-60 years old. Flu-like symptoms are often testified. As Alpine neighbours to the Milan group, we would report that, besides this typical presentation, a second distinctive presentation is found in high-altitude mountaineers in our country.
Initial management and angiogram
Based on the description of the ECG and the low troponin level in this case, the coronary event is undoubtedly (hyper)acute. The initial management is fair (<180 minutes after onset of pain) given that the presentation was unusual (atypical pain, dry cough over the last few days). Based on the single non-moving view that we received, the coronary angiogram confirms a subocclusive dissection of the shaft of the left main coronary with “train rail” sign, and impaired coronary flow (TIMI 1). The dissection flap seems to be confined to the left main, sparing both branches with decent anastomosis segments in case of coronary artery bypass grafting (CABG) surgery. We would classify this subocclusive dissection as type F in the NHLBI classification1 and type I in the “simplified” classification for LM dissection2. Coronary haemodynamic impairment (and perhaps also already systemic?) certainly portends significant morbidity and mortality for this patient2,3. Therefore, two options remain: percutaneous coronary intervention (PCI) and CABG.
Coronary revascularisation
The choice of the initial strategy depends mainly on three factors: the patient’s haemodynamic stability or instability (systolic blood pressure <90 mmHg with signs of systemic hypoperfusion), PCI operator skills, and the availability of a cardiac surgical team. The main risks of CABG surgery are: (a) the time necessary to organise the operation theatre and the staff (surgeon/anaesthetist/perfusionist; often >90 minutes from angiogram to cut-time), which might be fatal in case of obstructive SCAD; and (b) the hurdles to differentiate the true from the false lumen in case of extensive SCAD (Figure 2). The main risks of PCI are: (a) an unacceptable end result (haematoma propagation, stenting of the false lumen); and (b) an increased risk of late complications (restenosis or stent thrombosis that might present with sudden cardiac death) in comparison to CABG surgery. As is often the case, these two initial strategies are not mutually exclusive. It can happen, for example, that CABG is considered as an initial strategy (extensive dissection without involvement of the anastomotic segments) but, due to circulatory collapse, an emergency PCI is performed. Conversely, in case of an unacceptable result after an initial PCI4, a transfer for CABG surgery can be considered.
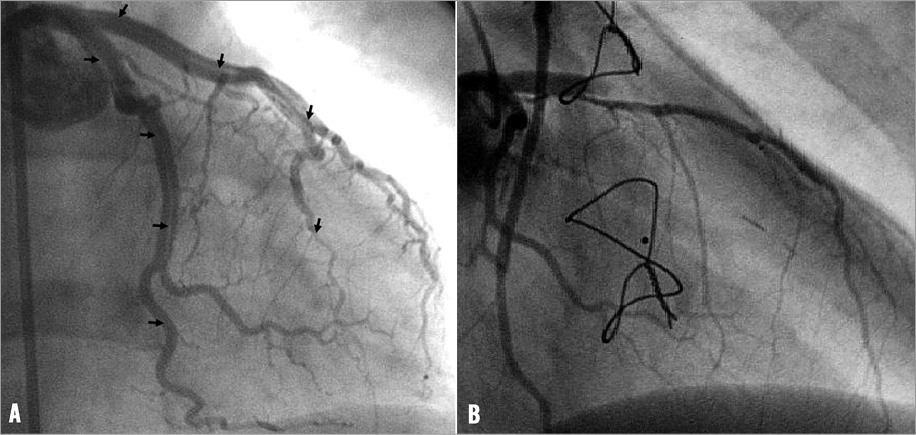
Figure 2. Angiographic example of patient treated by coronary artery bypass grafting (CABG) surgery for extensive dissection (arrowheads) from the left main into both major epicardial branches.
How would I treat?
Given the obstructive nature of the dissection, together with slow coronary flow, a large area at risk, and a previous episode of arterial hypotension, we would have expected a rapid haemodynamic deterioration in the present case. Accordingly, we would have informed the intensive and on-call surgical cardiac teams and would initially have chosen an interventional strategy. Ideally, we would like to have a percutaneous left ventricular assist device or ECMO available in case of bail-out need (cardiac arrest) during PCI.
The practical aspects of the PCI are left at the discretion of the operator. We would probably have chosen a catheter with a short tip to prevent any conflict with the dissection, and two guidewires, at least one with hydrophilic coating with accurate torquability and improved gliding capacity in order to advance the wires precisely and gently. Intracoronary nitroglycerine should be given to alleviate any coexisting coronary spasm. Once both major epicardial branches have been wired, we would certainly confirm the passage of one or other guidewire into the true lumen with intravascular imaging. Intravascular imaging permits a “full picture” of the dissection by identifying true and false lumens, length of double lumen, size of intramural haematoma, presence of intimomedial flap, associated thrombus, or even the entry tear (Figure 3).
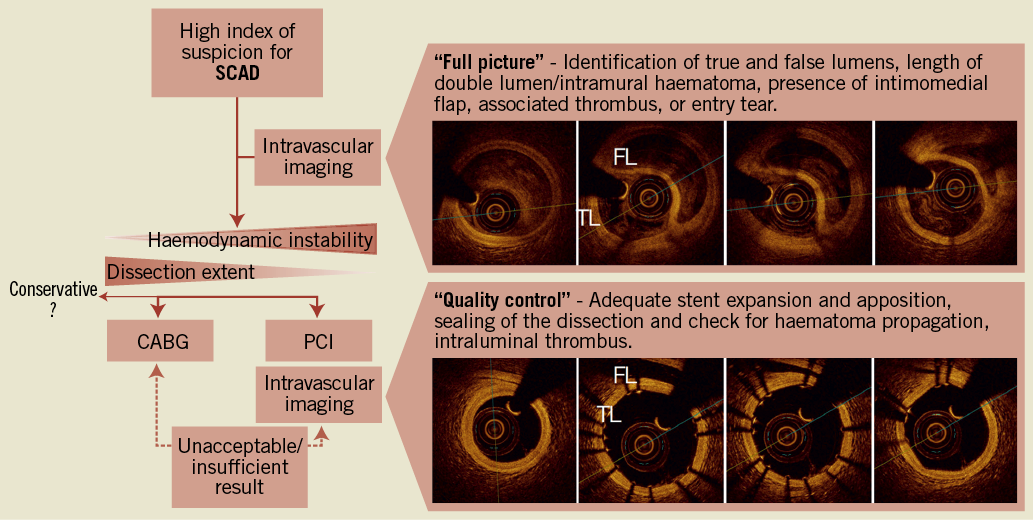
Figure 3. Clinical decision-making schema in the clinical management of spontaneous coronary artery dissection (SCAD). PCI that is performed more quickly and CABG that has better long-term outcome are not mutually exclusive, and might be considered sequentially in up to 10% of such cases. CABG: coronary artery bypass graft surgery; PCI: percutaneous coronary intervention
While we were reluctant to use the optical frequency domain imaging (second-generation OCT, OFDI) in this particular setting in the past, two positive experiences and the recent publication of Alfonso and colleagues5 have convinced us that OFDI imaging could be carried out in these situations. However, since the image acquisition requires forced injection of contrast, OFDI imaging can be harmful by allowing a rapid expansion of the haematoma together with propagation of the dissection. Therefore, in our view, intravascular ultrasound (IVUS) remains the examination of choice.
Once the initial intravascular imaging reveals the proper positioning of the wire into the true lumen, the operator should try to seal off the false lumen with a stent. Of course, an adequate and timely antithrombotic regimen should be given.
Since drug-eluting stents have demonstrated better long-term clinical outcome than bare metal stents after left main PCI for acute coronary syndrome6, as well as in cases of left main dissection2, we would certainly implant a 3.5-4.0/18-22 mm second or third-generation DES. We would choose a stent a bit longer than the visible haematoma since the haematoma might propagate during inflation. Furthermore, we would make a long coronary inflation (>60 seconds) and run a second coronary imaging (“quality control”) in order to check for adequate stent expansion and apposition, efficient sealing of the dissection, haematoma propagation, and intraluminal thrombus. An optimal post-procedural result is the keystone of percutaneous management. In case of a suboptimal result, discussion with a heart surgeon would have been mandatory.
Conflict of interest statement
S. Cook receives research grants from Boston Scientific and Biosensors. M. Togni has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
The case concerns a 45-year-old female smoker with a family history of coronary artery disease, presenting with the clinical and angiographic manifestation of a spontaneous coronary artery dissection (SCAD). This is a rare cause of acute coronary syndromes with a striking predilection for young female patients7. Several aspects should be evaluated when confronted with this patient population.
First, the anticipated pathophysiology of the angiographical image should preferably be confirmed using intravascular imaging techniques. Intravascular ultrasound (IVUS) is the classical imaging modality showing in most cases intramural haematoma with minimal or no atherosclerosis8. More recently, optical coherence tomography (OCT) has been shown to comprise precise visualisation of the intimal tear and assessment of the exact location of the intramural haematoma, unlike existing IVUS systems5. Besides confirming the diagnosis, this technique is of great value not only for understanding the problem, but also for visualising the origin of the dissection and the extent and length of the lesion. Besides, it can be used for guiding a correct wiring position and for allowing accurate geographical positioning and optimal deployment if using stents.
After ensuring the correct diagnosis, the right treatment strategy is the second important issue to address. No guidelines are available concerning the management of patients with SCAD. In general, patients can be treated medically, using percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG). Decision making should be done, based upon both clinical and angiographical factors9. When no signs of ongoing ischaemia or haemodynamic instability are present, conservative management is defensible in small and medium-sized vessels, since complete resolution has been observed after (long-term) follow-up10. Medical treatment in general is analogous to the treatment of acute coronary syndromes, including antithrombotic therapy with (low molecular weight) heparin, aspirin and ADP inhibitors (clopidogrel or the second-generation inhibitors prasugrel and ticagrelor). However, aggressive antithrombotic therapy may theoretically also cause an increase of the intramural haematoma, as has been shown in the past using fibrinolysis11. In cases of marked major epicardial flow impairment, PCI or alternatively CABG could be considered.
After ensuring restoration of epicardial flow in the acute phase, additional investigations should be performed to elucidate the cause of the event. Two connective tissue diseases in particular are known to be associated with SCAD, namely Marfan’s syndrome and Ehlers-Danlos syndrome type IV12,13. Other related conditions to exclude are pregnancy, systemic lupus erythematosus vasculitis or cocaine abuse.
Given the fact that in the described case a subocclusive, flow-limiting dissection of the left main was present, it may well be that the treatment of choice will in the end involve stenting. However, it may be wise to start by giving nitroglycerine, since diffuse spasm could mimic an angiographic image of dissection, particularly since there was also a diffuse reduction of the lumen of the left anterior descending, intermediate and left circumflex. After ruling out an important spasm component, sometimes only wiring the vessel may be sufficient to restore flow into the true lumen. If an important luminal reduction remains, stenting is the treatment of choice. IVUS and/or OCT may help guide the interventionalist to find the entry site of the dissection. If the dissection is more diffuse and distally located, CABG could be an alternative; however, the surgeon might have a hard task finding the true lumen for the anastomosis.
Despite the fact that mortality in the acute phase may be relatively high (in the post-partum SCAD up to 38%)14, it is also known that the clinical course thereafter is relatively benign15. A multicentre prospective registry with a case-control group is ongoing and may identify the role of different therapeutic strategies in this unusual pathologic condition16.
Conflict of interest statement
The authors have no conflicts of interests to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
In consideration of the clinical presentation (STEMI) and after discussion with our cardiac surgeons we decided to proceed quickly with percutaneous coronary intervention.
After elective insertion of an intra-aortic balloon pump (Arrow International, Reading, PA, USA), the left main was engaged, via the right femoral route, with a 6 Fr XB 3 guiding catheter (Cordis Corporation, Miami, FL, USA). Unfractionated heparin was administered and the activated coagulation time (ACT) was 276 seconds. The true lumens of the first diagonal and left circumflex arteries were initially wired using Balance Middleweight wires (Abbott Vascular, Santa Clara, CA, USA). Predilatation of the left main was performed using a 2.5×20 mm Maverick balloon (Boston Scientific, Natick, MA, USA) with improvement of coronary flow. The first image after predilatation showed a long dissection involving the left anterior descending (LAD), first diagonal, intermediate branch (IR) and circumflex arteries (LCX) (Figure 4, Moving image 2). After wiring the true lumen of the LAD we decided to stent only the entry point of the dissection in the left main using a provisional T-stenting technique. A 3.0×28 mm drug-eluting stent (Nobori; Terumo Corporation, Tokyo, Japan) was implanted at 10 atm in the left main into the LAD (Moving image 3) with complete sealing of the dissection flap. Intracoronary nitrates were administered. Final TIMI 3 flow was achieved in the left system despite residual dissection in the branches beyond the stent (Figure 5, Moving image 4 and Moving image 5).
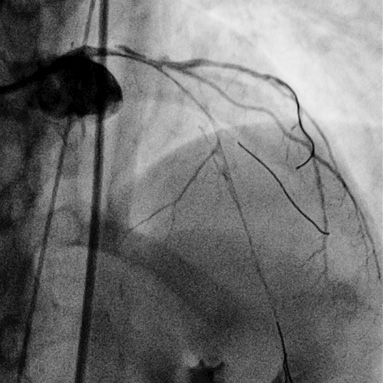
Figure 4. Coronary angiography after predilatation of LM and LAD shows a long dissection involving the left anterior descending artery (LAD), first diagonal, intermediate branch (IR) and circumflex arteries (LCX).
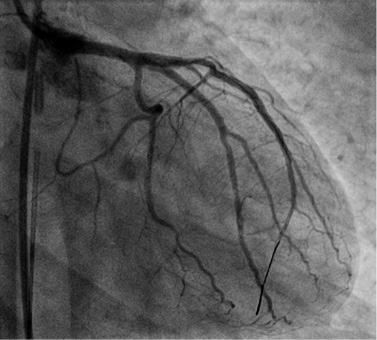
Figure 5. Final results after PCI and stenting and administration of nitrates: complete sealing of the dissection flap, final TIMI flow grade 3 in the left system despite a residual dissection in the LAD, intermediate and LCX branches.
The patient’s hospital course was uneventful. Cardiac enzymes peaked at 12 hours from the procedure with a CK-MB fraction of 15.6 mcg/L and a troponin I of 2.05 mcg/L. Serological studies and exams excluded secondary forms and predisposing factors associated with coronary artery dissection. The echocardiogram showed mildly depressed left ventricular ejection fraction (48%) and akinesis confined to the anterior and apical walls of the left ventricle. In-hospital cardiac CT performed one week after PCI and stenting confirmed the elimination of the dissection flap in the proximal left main after stenting and the presence of complete thrombosis of the false lumen and good perfusion of the true lumen in the LAD, LCX and intermediate branches (Figure 6). The patient was discharged on dual antiplatelet therapy (aspirin 100 mg and prasugrel 10 mg per day) in addition to treatment with ACE inhibitors, atorvastatin and ivabradine.
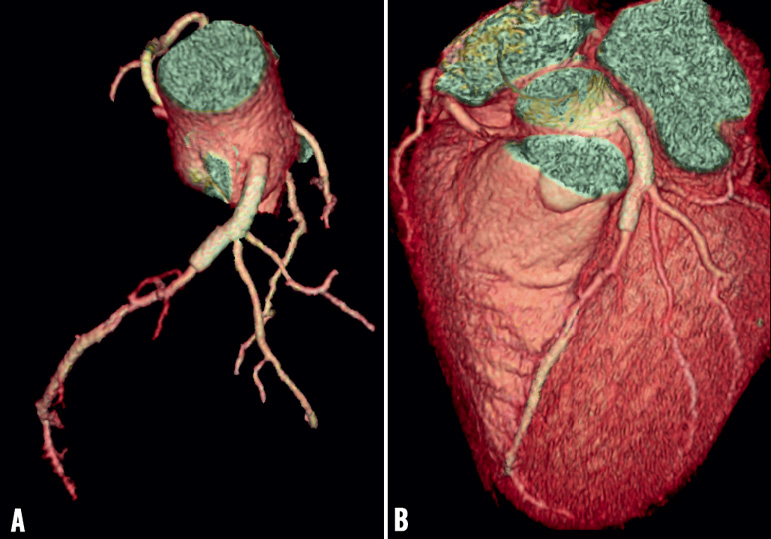
Figure 6. Three-dimensional reconstruction of coronary CT showed sealing of the proximal part of the left main after stenting and the presence of complete thrombosis of the false lumen with good perfusion of the true lumen in the LAD, LCX and intermediate branches.
Discussion and conclusion
Spontaneous coronary artery dissection (SCAD) remains today a rare, intriguing and challenging clinical entity9,15. In the present case, we describe a spontaneous dissection extending from the LM artery into the LAD, IB and LCX arteries in the setting of STEMI. Two major issues need to be taken into consideration when deciding on the possible therapeutic options:-
Which revascularisation strategy to choose: CABG or PCI?
The revascularisation strategy is clearly influenced by the patient characteristics and clinical presentation as well as the angiographic findings and extent of dissection3.
Symptomatic patients with STEMI and haemodynamic compromise should be treated with an emergency myocardial revascularisation strategy. Immediate restoration of blood flow is imperative to ensure patient survival and to minimise the chance of further dissection. In this setting, primary PCI should be the therapy of choice. Indeed, controversy exists regarding management of SCAD initially treated medically9,15,16. Some authors suggest the use of PCI and stenting in cases of single vessel or two vessel dissection and the use of CABG in the presence of multivessel and left main involvement17-21. However, finding the true lumen during bypass surgery could be technically challenging, and extensive dissection involving distal vessels may preclude surgery due to poor distal targets, as in our patient.
If PCI is the treatment of choice, which strategy to follow?
There are two possible strategies in SCAD: seal only the entry point or the entire dissection.
The first option is to adopt a single stent or “spot stent” approach in order to seal only the entry point of the dissection. Because in our case (SCAD in STEMI) the objective was to restore the coronary flow promptly, our choice was to seal the entry point and proceed with only one stent.
A second option could have been to proceed with a complete reconstruction of the coronary tree with multiple stents and consequently a “full metal jacket”. This approach could compromise side branches, resulting in periprocedural myocardial infarction. In addition, this strategy may be complicated by a relatively high risk of restenosis and stent thrombosis due to the number of stents implanted22,23. It also requires long-term dual antiplatelet therapy. Furthermore, the use of extensive stenting in the distal vessel segments can clearly limit the possibility of performing CABG in the future.
The optimal treatment of SCAD remains controversial. PCI and stenting are safe and effective for the treatment of left main and multivessel spontaneous dissections especially in the setting of STEMI. The spot or single stent approach to seal the entry point might be the preferred strategy to prevent further propagation and to restore true lumen patency.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Baseline coronary angiography showing a coronary dissection in the left main and diffuse reduction of the lumen involving the LAD, IR and LCX arteries.
Moving image 2. Angiography after wiring LAD, LCX and diagonal branches showed the extent of dissection involving the whole of the left coronary system.
Moving image 3. Initial angiographic result after PCI and stenting of the left main and LAD.
Moving images 4 and 5. Final results after intracoronary nitrates showed a TIMI 3 flow in the left system and complete sealing of the dissection flap with residual dissection.

