Abstract
Background: Spontaneous coronary artery dissection (SCAD) is an increasingly diagnosed cause of myocardial infarction. Although different SCAD angiographic classifications exist, their clinical impact remains unknown.
Aims: The aim of this study was to evaluate the relationship between an angiographic classification and the development of adverse clinical events during the follow-up of a large, unselected cohort of patients with SCAD.
Methods: We conducted an observational study of consecutive SCAD patients from 26 centres across Italy and Spain. Cases were classified into five different angiotypes according to the latest classification endorsed by the European Society of Cardiology. The main composite endpoint included all-cause death, non-fatal myocardial infarction (MI), and any unplanned revascularisation.
Results: In total, 302 SCAD patients (mean age 51.8±19 years) were followed up for a median of 22 months (IQR 12-48). At 28 days, the composite outcome was higher for the angiotypes with a circumscribed contained intramural haematoma (2A and 3): 20.0% vs 5.4%, p<0.001 (non-fatal MI: 11.0% vs 3.5%, p=0.009; unplanned revascularisation: 11.0% vs 2.5%, p<0.001). This was sustained during follow-up (24.5% vs 9.9%, p=0.001). There were no differences in mortality (0.3% overall). The presence of an angiotype 2A or 3 was an independent predictor of a higher incidence of the composite outcome (adjusted HR 2.44, CI: 1.24-4.80, p=0.010).
Conclusions: The SCAD angiographic classification correlates with outcome. Those presenting with an angiographically circumscribed contained intramural haematoma (angiotypes 2A and 3) showed an increased risk of short-term adverse clinical events that was maintained during follow-up.
Introduction
Spontaneous coronary artery dissection (SCAD) is a condition that predominantly affects middle-aged women, causing myocardial infarction (MI), cardiac arrest, and cardiac death1. SCAD can be defined as the acute and spontaneous development of a false lumen within the coronary artery wall that leads to flow limitation by compression of the true coronary lumen (with this definition excluding dissections caused by complicated atherosclerotic disease, iatrogenic factors or related to direct trauma)2. Although SCAD has largely been overlooked as a cause of acute coronary syndromes, the expanded use of coronary angiography and intracoronary imaging techniques in patients presenting with acute coronary syndrome has led to an increased awareness of this condition3.
From a pathological perspective, the hallmark of SCAD is the intimomedial flap that separates the true and false lumens. Two different patterns have been identified: the communicated double lumen – fenestrated SCAD – and the contained intramural haematoma (IMH) – non-fenestrated SCAD1. On the other hand, the angiographic manifestation of these two pathological substrates is more diverse. In a study by Waterbury et al on conservatively managed SCAD, angiographic presentation as IMH entailed a greater risk of early clinical progression compared to the angiographic radiolucent flap and linear double lumen (intimomedial tear with communication between false and true lumens) after adjusting by several confounders4. García-Guimaraes et al reported similar findings from a large multicentre registry, in which type 2 SCAD, defined by the presence of long contained IMH (>20 mm), resulted in being an independent predictor of in-hospital MACE5.
Different classifications have been developed to depict the rich angiographic spectrum of SCAD2,6,7, which has contributed to the knowledge of this entity. However, we are still uncertain whether the use of these classification systems has clinical or therapeutic implications. In this study, we aimed to evaluate the relationship between an angiographic classification and the development of adverse clinical events during the follow-up of a large, unselected cohort of patients with SCAD.
Methods
STUDY DESIGN AND POPULATION
DISCO-IT/SPA (DIssezioni Spontanee COronariche Italian-SPAnish) (ClinicalTrials.gov identifier: NCT04415762) was an observational, international, multicentre, retrospective registry which enrolled SCAD patients from 23 centres in Italy and Spain. Details regarding eligibility and data collection are provided in Supplementary Appendix 1.
ANGIOGRAPHIC ANALYSIS AND CLASSIFICATION
Diagnosis confirmation and subsequent classification into five angiographic categories or angiotypes (Figure 1) was performed through a two-step core lab process. A detailed description is provided in Supplementary Appendix 2.
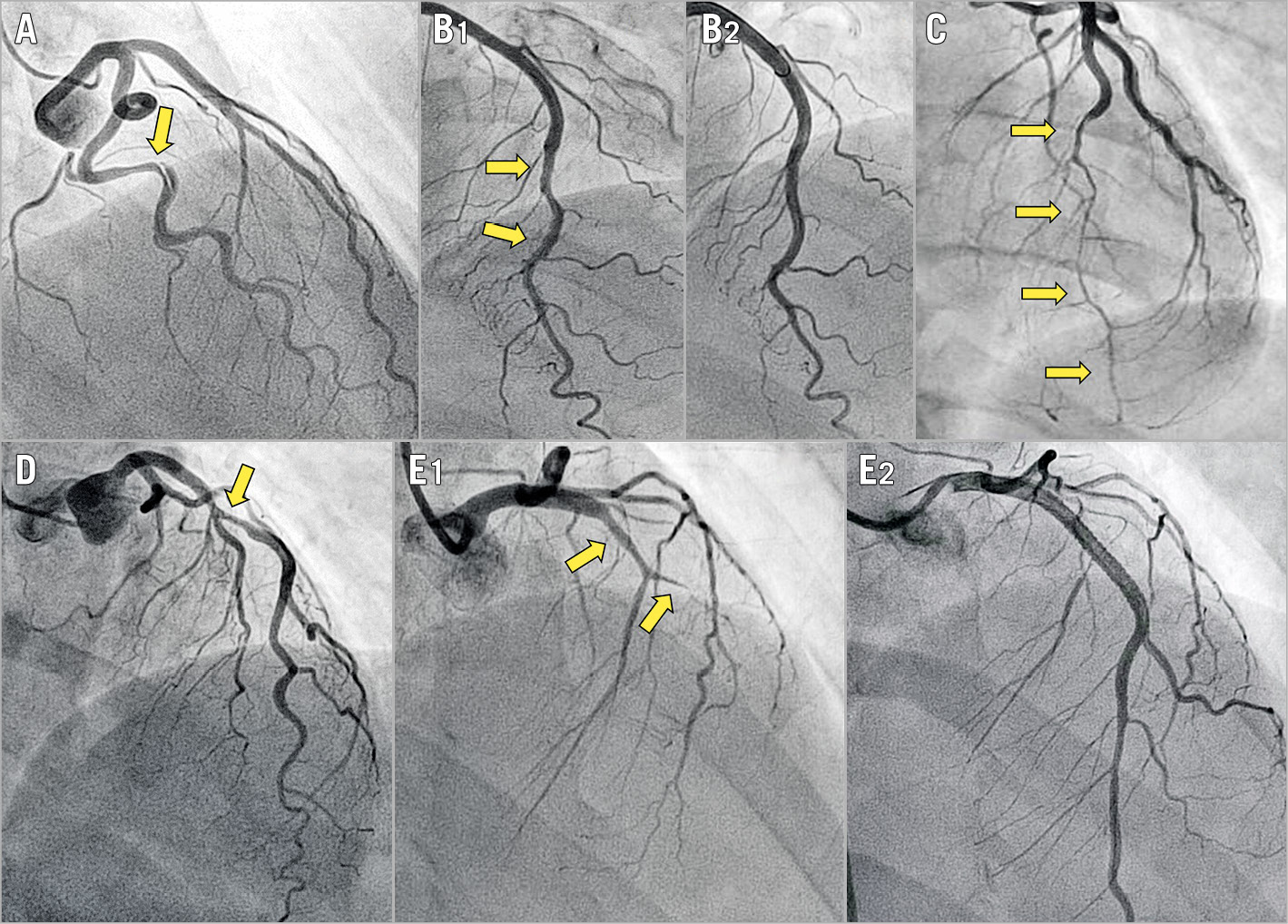
Figure 1. Angiographic classification of SCAD. Arrows indicate the dissected segments. A) Angiotype 1, radiolucent flap or dual lumen. B1) Angiotype 2A, long (>20 mm) narrowing with distal calibre recovery. B2) Vessel restoration three months after conservative management of B1. C) Angiotype 2B, long (>20 mm) narrowing involving the main distal vessel. D) Angiotype 3, focal (<20 mm), sometimes tubular stenosis. E1) Angiotype 4, total occlusion of the main vessel. E2) Flow restoration after stenting.
FOLLOW-UP AND OUTCOMES
Patients were followed up routinely after discharge by telephone/office contact at 1, 6, and 12 months, and annually thereafter. Follow-up was censored at four years. Clinical outcomes included all-cause death, non-fatal MI (fourth universal definition of myocardial infarction8), any unplanned revascularisation, stroke, or Bleeding Academic Research Consortium (BARC) bleeding events. Only BARC type 2 or greater bleeding events were considered. The latter outcome was only collected for the first four weeks. The primary outcome was a composite of major adverse cardiovascular events (MACE), which consisted of all-cause death, non-fatal MI, and any unplanned revascularisation. MACE were analysed and reported at 28 days (4 weeks) and at a maximum of 4 years of follow-up. Percutaneous coronary intervention (PCI) success was defined as Thrombolysis In Myocardial Infarction (TIMI) flow 2-3 with residual stenosis <30% (after stent/scaffold implantation) or <50% (after simple balloon angioplasty).
STATISTICAL ANALYSIS
This section is reported in Supplementary Appendix 3.
Results
PATIENT CLINICAL CHARACTERISTICS
We retrospectively enrolled 302 patients with complete follow-up at a minimum of six months. Overall, mean age was 51.8±10 years (range 23-84 years) and there was a female preponderance (88.4%). Among women with SCAD, 13.2% were receiving hormonal therapy. No significant differences were noted among the different groups with regard to baseline characteristics (Table 1), except that more patients with angiotype 1 presented with a recent pregnancy and that more patients with angiotype 2B presented with a post-menopausal status. With regard to clinical presentation (Table 2), 48.0% presented with ST-elevation MI (STEMI) and 45.0% with non-ST-elevation MI (NSTEMI). Patients with angiotype 4 (TIMI 0) presented more often with STEMI than the other angiotypes (71.6% angiotype 4 vs 39.3% non-angiotype 4, p<0.001).
ANGIOGRAPHIC FEATURES, MANAGEMENT, AND PROCEDURAL OUTCOME
Angiographic characteristics are described in Table 2. Half of the 302 patients included had angiotype 2 (49.3%), these being 26.5% 2A and 22.8% 2B. The other half comprised angiotype 4 (26.8%), followed by angiotype 1 (17.2%) and, lastly, angiotype 3 (6.6%). The majority of SCAD involved a single coronary artery territory (88.1%), the most common one being the left anterior descending artery and/or its branches (54.3%). However, angiotype 3 was seen more often in the left circumflex artery (65%). Mean angiographic stenosis was 85.7% (SD±17.4%) and mean dissection length was 40.3 mm (SD±24.3 mm). Procedural characteristics and management are described in Table 3. Most patients (n=198, 65.6%) were managed conservatively as the initial strategy, with significant differences among angiotypes (more revascularisation in angiotypes 1 and 4). Intracoronary imaging was used in 27.8% (n=84) of the patients. Optical coherence tomography (OCT) was used more frequently in patients with angiotypes 2A and 3 (22.0% vs 10.3%, p=0.03). Moreover, 100 patients underwent ad hoc PCI (33.1%), 78% of which were adjudicated as successful in the core laboratory. Common complications included false lumen stenting and proximal and/or distal dissection propagation. Four patients (1.3%) underwent coronary artery bypass graft (CABG) surgery as first therapeutic option.
SHORT- AND LONG-TERM CLINICAL EVENTS
The median hospital stay was 6 (IQR 5-8) days. At the time of discharge, a total of 120 patients (39.7%) had undergone revascularisation (38.4% PCI and 1.3% CABG). Most patients were discharged home on aspirin (96.6%), and P2Y12 inhibitors (76.8%) – with 75.5% on a dual antiplatelet regimen, beta-blockers (81.3%) and statins (70.5%). Supplementary Table 1 shows the pharmacological treatment according to the initial management strategy; there were no differences except in antiplatelet treatment. Table 4 shows clinical outcomes during the first 28 days and in the long term. The occurrence of 28-day MACE was significantly different among angiotypes, mainly due to a higher incidence in patients with a circumscribed contained IMH (angiotypes 2A and 3) than in the others (20.0% vs 5.4%, p<0.001), particularly in the first two weeks (Figure 2). This was driven mainly by an increase in non-fatal MI (11.0% vs 3.5%, p=0.009) and unplanned revascularisation (11.0% vs 2.5%, p<0.001) in those patients. Among patients undergoing unplanned revascularisation, chest pain with evidence of ischaemia on the electrocardiogram (ECG) was the most common cause (n=14, 87.5%), while angiographic progression of the initial dissection with flow worsening was the most common finding (n=13, 81.3%). Of this latter subgroup, the majority (n=8, 61.5%) were initially managed conservatively.
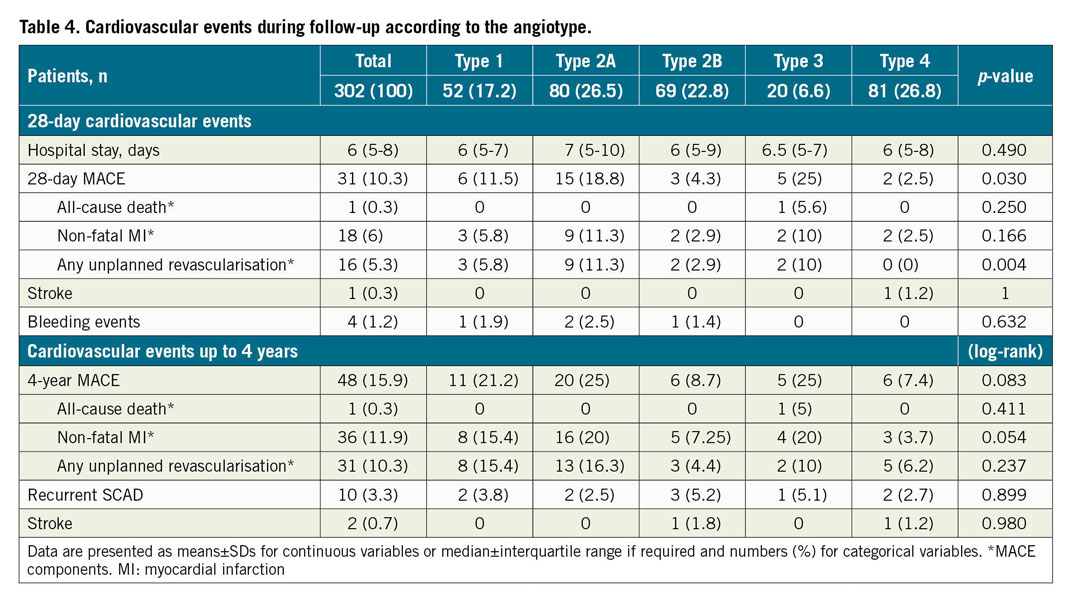
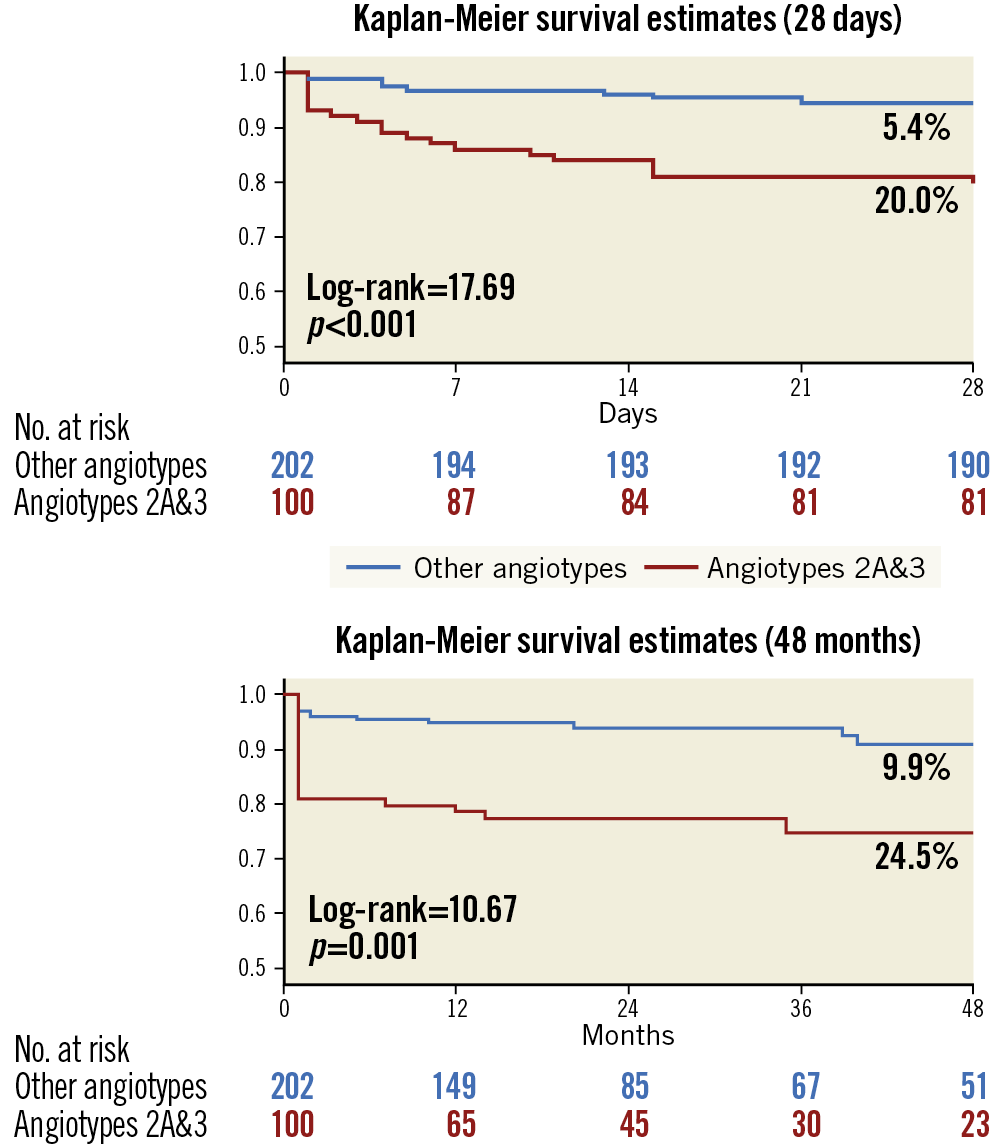
Figure 2. Kaplan-Meier curves for MACE in angiotypes 2A and 3 versus other angiotypes during the first 28 days and long-term follow-up.
Patients were followed up for a median of 22 months (minimum 6 months, maximum 4 years, IQR 12-48 months). There was only one death that occurred during hospitalisation in a patient admitted with cardiac arrest (cardiac death). MACE occurred in 48 (15.9%) patients in the whole cohort with a different incidence among the five angiotypes (Table 4, Supplementary Figure 1): angiotype 1: 21.2%, angiotype 2A: 25.0%, angiotype 2B: 8.7%, angiotype 3: 25.0% and angiotype 4: 7.4% (log-rank=13.30, p=0.010). The excess of MACE observed in patients with angiotype 2A and 3 compared with the rest was maintained in the follow-up: 24.5% versus 9.9% (log-rank=10.67, p=0.001) (Figure 2). Looking at the MACE components, non-fatal MI was significantly higher in angiotypes 2A and 3 (20.0% vs 7.9% in other SCAD angiotypes, log-rank=8.55, p=0.003) as was unplanned revascularisation (15.0% vs 7.9%, log-rank=4.26, p=0.039), with no differences in mortality (Supplementary Figure 2). On the other hand, angiotype 4 had a lower rate of MACE compared to the other angiotypes (7.4% vs 19.0%, p=0.015).
After multivariate adjustment in Cox regression analysis, circumscribed IMH (types 2A and 3) was confirmed as an independent predictor of MACE (adjusted hazard ratio [HR] 2.44, 95% CI: 1.24-4.80, p=0.010). Conversely, single antiplatelet therapy was identified as a protective factor (HR 0.31, 95% CI: 0.11-0.87, p=0.027) (Supplementary Table 2). MACE rates according to the initial therapeutic strategy and each angiotype during follow-up are reported in Supplementary Table 3. No significant differences were found between the revascularisation strategy and the conservative management groups (18.3% vs 14.7%, respectively, p=0.426); however, in the angiotype 4 subgroup there was a higher incidence of events in those who underwent revascularisation (13.9% vs 0%, p=0.027).
Discussion
In this multicentre, international study we found that angiographic presentations of SCAD suggesting a circumscribed contained IMH– angiotypes 2A and 3 – showed a higher incidence of MACE, notably during the first 28 days (including hospital admission). This excess of adverse events relates mainly to recurrent MI and unplanned PCIs during hospitalisation, being unrelated to initial treatment (conservative vs revascularisation).
SCAD is increasingly recognised as an important cause of acute coronary syndrome among young and middle-aged women. The demographic data derived from this large SCAD series are very much in keeping with the most recent and largest reports from other registries and countries5,9,10, confirming again that SCAD presents predominantly in middle-aged patients, with a female preponderance and few, but not absent, cardiovascular risk factors. Mood disorders such as anxiety or depression were frequent, as were hormonal stress situations. The clinical presentation, essentially as a classic acute coronary syndrome, was often accompanied by an identifiable triggering stressor. STEMI was the predominant presentation in cases of SCAD type 4, as expected. In keeping with other SCAD angiographic series, angiotype 3 was the least common presentation, probably underrepresented owing to underdiagnosis.
This article informs on detailed short- and long-term outcomes in patients with a confirmed SCAD diagnosis. Survival to discharge and during follow-up was particularly good, with only one in-hospital cardiac death (mortality 0.3%). With the majority being treated conservatively as the initial treatment strategy, most patients were discharged without MACE. Of note, in-hospital recurrent MI occurred in 6.0% and any unplanned revascularisation in 5.3%, highlighting the importance of monitoring for recurrent ischaemia which requires urgent revascularisation in these patients.
In our study, patients with an angiographic SCAD presentation that denotes the presence of circumscribed haematoma with preserved patency in the distal vessel (angiotypes 2A and 3) – as opposed to those with double lumen (angiotype 1), distal haematoma (angiotype 2B) or blocked vessel (angiotype 4) – underwent unplanned revascularisation due to reinfarction during admission more often. We opted to merge angiotypes 2A and 3 into one single group based on a similar pathological substrate (contained haematoma circumscribed to a vessel segment) and on similar clinical behaviour. In the Spanish national SCAD registry, García-Guimaraes et al5 found that IMH was an independent predictor of in-hospital events. Similarly, Waterbury et al4 reported data from the Mayo Clinic registry showing that patients with SCAD experiencing clinical progression over hospital admission frequently presented initially with isolated IMH (without intimal dissection).
Circumscribed IMH is an unstable structure that may progress in length and degree of luminal obstruction, causing clinical ischaemia. It has been suggested that fenestrated and non-fenestrated spontaneous dissections may be two distinct pathological manifestations of the same substrate11. Hypothetically, an initial haemorrhage leads to different angiotypes that correspond to distinct stages of the natural history of SCAD. Thus, when a focal IMH starts the process, this may result in either an intimal rupture (angiotype 1) or a contained haematoma, which can be circumscribed (angiotype 3 or 2A) or propagated through the distal vessel (angiotype 2B). Furthermore, any of these may potentially occlude the vessel (angiotype 4). We hypothesise that an angiographically circumscribed IMH may correspond to an early pathological phase of SCAD and, for this reason, would suggest a more unstable lesion with higher likelihood of changing, including progression into a more severe stenosis or vessel occlusion that can cause recurrent ischaemia, non-fatal MI and may lead to unplanned revascularisation. Supporting this hypothesis, the observed protective role of single antiplatelet therapy compared to a dual antiplatelet regimen may be explained by a lower risk of IMH progression. For these reasons, this subset of patients may benefit most from a prolonged admission with close surveillance and monitoring.
Patients with angiotype 1 SCAD also showed a high rate of adverse events in this series (21.2%). We believe that this finding may carry a degree of temporal bias. As a result of the fact that a radiolucent flap was virtually the only angiographic feature used to diagnose SCAD, earlier cases in our series belong mostly to angiotype 1 (diagnosis dates are shown in Table 1). Besides, as conservative management of SCAD did not become the standard of treatment until recently, these earlier angiotype 1 SCAD cases were treated more frequently with PCI (PCI rate of 45%). Of note, early angiotype 1 cases had proximal vessel involvement more often than those with other angiotypes (32.7% vs 14.1%, p=0.01), which after all reflects a higher-risk cohort. The limited spatial resolution of angiography may add some classification bias here, i.e., small intimomedial flaps are less likely to be detected in mid and distal segments and therefore those lower-risk cases may be classified into other angiotypes.
Interestingly, patients with an angiotype 4 SCAD showed the best MACE-free survival curve, with a low event rate during admission and long-term follow-up. This association was significant despite being the group with the highest rate of PCI (53.8%), with no events in those managed conservatively. A more favourable evolution of these patients may be explained by the fact that angiotype 4 constitutes an advanced phase of the dynamic process of SCAD in some cases, with already established MI and free from the instability of angiotypes 2A and 3. Distal or secondary branch involvement was predominant in most cases with angiotype 4 which implies a limited extension of the necrosis, whereas in those cases with more proximal occlusions and a significant territory at risk patients would receive immediate revascularisation.
Along the same lines, patients with angiotype 2B SCAD also showed a low incidence of MACE, all these cases involving predominantly distal segments of a coronary artery or branch. The latter highlights the importance of breaking down angiotype 2 into 2A and 2B, which gives a clear sense not only of diagnosis but also of prognosis, and even questions the sense of grouping these different presentations under the same category. Based on our findings, the angiographic classification of SCAD could be simplified to reflect the clinical significance of the various angiotypes.
Moreover, despite the fact that intracoronary imaging has an important role for diagnosing SCAD when angiography is inconclusive, these techniques should be used sensibly in this clinical scenario. We believe that the relatively low usage of intracoronary imaging reported in this study (27.8%) and in other large contemporary cohorts5,9 testifies to an increasing acquaintance with the characteristic angiotypes of SCAD as well as to the understanding of the risks of coronary instrumentation in these patients12.
Taken together, we found that patients with SCAD presenting with angiographic patterns suggesting a circumscribed contained IMH were more prone to present in-hospital reinfarction and to require urgent unplanned revascularisation. This association with worse outcomes was maintained in the long-term follow-up and was demonstrated to be independent after multivariable adjustment. The data presented support a strategy of prolonged admission and monitoring in those SCAD patients presenting with high-risk angiotypes. In general, a clinically guided approach should be followed. However, for the surveillance of selected patients with proximal vessel involvement or proximal residual stenosis following PCI, computed tomography is our preferred imaging modality. The implications of these findings for tailoring treatment in patients with SCAD need to be elucidated in future studies.
Limitations
This study has the limitations of a retrospective analysis. Given the shifts in diagnosis and therapies of SCAD since 2009, an inherent temporal bias may potentially affect the results of this study. The clinical indications that may have accounted for the decision on revascularisation were not protocolised, and therefore probably heterogeneous among the different participating centres. Furthermore, the angiography-based definition of “contained IMH” is somewhat imprecise as small fenestrations of the intimomedial lamina (disclosable by pathology or OCT) cannot be excluded. Notwithstanding this, this study was designed to evaluate an angiography-based classification and therefore its findings are applicable to the mere use of this imaging modality. Finally, this study was not designed or powered to assess the impact of treatment strategies among the different angiotype groups. Future studies are needed to assess this matter.
Conclusions
The use of an angiographic classification of SCAD discriminated patients with different rates of events. Patients with SCAD presenting with an angiographically circumscribed and contained haematoma (angiotypes 2A and 3) showed an increased risk of adverse clinical events during admission that was maintained during long-term follow-up.
|
Impact on daily practice SCAD may manifest in a variety of angiographic patterns (angiotypes). Those with a circumscribed intramural haematoma were found to be at higher risk of adverse clinical events in the short term, driven by a fourfold increased risk of unplanned revascularisation and a threefold greater risk of reinfarction. These poorer outcomes were sustained during long-term follow-up. These patients may benefit from prolonged monitoring and hospital stay to identify early ischaemic relapses. |
Appendix. Study collaborators
Ivan Núñez-Gil, PhD, Hernán Mejía-Rentería, PhD, Luis Nombela-Franco, PhD, Pilar Jiménez-Quevedo, PhD, Gabriela Tirado-Conte, MD, Antonio Fernández-Ortiz, PhD, Carlos Macaya, PhD; Hospital Clínico San Carlos, IdiSSC, Universidad Complutense de Madrid, Madrid, Spain. Giorgio Quadri, MD, Francesco Tomassini, MD, Fabio Ferrari, MD, Fabio Mariani, MD, Alfonso Franzè, MD; Interventional Cardiology Unit, San Luigi Gonzaga University Hospital, Orbassano, and Rivoli Infermi Hospital, Rivoli, Turin, Italy. Matteo Bianco, MD, Laura Montagna, MD; Division of Cardiology, A.O.U San Luigi Gonzaga, Orbassano, Turin, Italy. Luca Lo Savio, MD; Division of Cardiology, Infermi Hospital, Rivoli, Turin, Italy. Angelica Rossi, MD, Bruno Loi, MD; Azienda Ospedaliera Brotzu, Cagliari, Italy. Gianmarco Annibali, MD; A.O. Ordine Mauriziano, Ospedale Umberto I, Turin, Italy. Massimiliano Scappaticci, MD; Santa Maria Goretti Hospital, Latina, Italy. Umberto Barbero, MD, Michele De Benedictis, MD; Ospedale Maggiore Ss. Annunziata - Savigliano (CN), Italy. Mario Iannacone, MD; Ospedale San Giovanni Bosco, Turin, Italy. Gianluca Campo, MD, Andrea Erriquez, MD; Azienda Ospedaliero Universitaria di Ferrara, Ferrara, Italy. Dario Buccheri, MD; Sant’Antonio Abate Hospital, Trapani, Italy. Chiara Cavallino, MD, Fabrizio Ugo, MD; Cardiology Division, Sant'Andrea Hospital, Vercelli, Italy. Primiano Lombardi, MD, Alessandra Truffa Giachet, MD; Cardinal Massaia Civil Hospital, Asti, Italy. Chiara Bernelli, MD, Annamaria Nicolino, MD; Santa Corona Hospital, Pietra Ligure (SV), Italy. Andrea Rognoni, MD, Marco Mennuni, MD, Giuseppe Patti, MD; Cardiology Department, Ospedale Maggiore Della Carita', Novara, Italy. Elisabetta Bordoni, MD; Ospedale Santa Maria della Misericordia, Perugia, Italy. Vincenzo Infantino, MD, Angelo Di Leo, MD; Division of Cardiology, Ospedale Civile di Ciriè (TO), Italy. Alfonso Gambino, MD, Giuseppe Pietro Greco Lucchina, MD; Santa Croce Hospital, Moncalieri (TO), Italy. Sebastian Cinconze, MD; Azienda Ospedaliera S. Croce e Carle, Cuneo, Italy. Giuseppe Musumeci, MD; A.O. Ordine Mauriziano, Ospedale Umberto I, Turin, Italy. Francesco Cassano, MD; Ospedale SS Annunziata, Taranto, Italy. Federico Beqaraj, MD, Luca Gaido, MD; Division of Cardiology, Maria Vittoria Hospital, Turin, Italy. Fabrizio D’Ascenzo, MD, Francesco Bruno, MD; Cardiology Department, AOU Città della Salute e della Scienza di Torino, Turin, Italy.
Funding
R. Mori received a training grant from the European Society of Cardiology (APP000019660). F. Macaya received a grant from Fundación Interhospitalaria para la Investigación Cardiovascular to conduct research on SCAD. The Fundación Interhospitalaria para la Investigación Cardiovascular provided financial support for the creation and maintenance of the SCAD online database at Hospital Clínico San Carlos and receives manuscript publication fees derived from related projects.
Conflict of interest statement
The authors/study collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

