Case summary
Background: A 42-year-old man with risk factors of hypertension and previous smoking was referred to our hospital five months after acute myocardial infarction due to spontaneous coronary artery dissection (SCAD) involving the left main coronary artery and the left anterior descending artery.
Investigations: Physical examination, electrocardiography, echocardiography, coronary angiography, intravascular ultrasound.
Diagnosis: Myocardial infarction due to SCAD.
Management: Percutaneous coronary intervention.
Keywords: IVUS, pseudoaneurysm, coronary artery dissection, acute myocardial infarction.
How should I treat?
Presentation of the case
A 42 year-old man with risk factors of hypertension and previous smoking was referred to our hospital for follow-up coronary angiography five months after spontaneous coronary artery dissection (SCAD). In June 2006 the patient had an acute anterior myocardial infarction (MI). Emergent coronary angiography performed in another centre showed SCAD involving the left main coronary artery (LM) and the proximal portion of the left anterior descending artery (LAD) with total occlusion at the mid-portion of LAD (Figure 1). The right coronary artery (RCA) was normal. The patient was conservatively treated with heparin, aspirin, clopidogrel and β-blocker. No primary percutaneous coronary intervention (PCI) or fibrinolysis was performed. He was discharged in stable condition with aspirin, clopidogrel, carvedilol and ramipril.
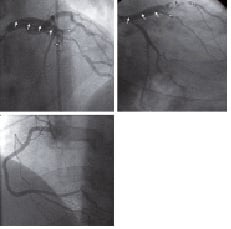
Figure 1. Basal coronary angiography shows a radiolucent flap in LM and the proximal portion of LAD (arrows) with distal occlusion (arrowheads). D1 (*) originates from the false lumen and D2 (**) originates from the true lumen with diffuse stenosis in the proximal segment. RCA was normal.
Since discharge he had been asymptomatic. He had no history of Marfan syndrome or Ehlers-Danlos syndrome. His physical examination was unremarkable. Electrocardiography showed Q-wave MI in the anterior wall. Echocardiography showed anteroapical hypokinesis of the left ventricle with ejection fraction of 45%.
Five-month follow-up angiography showed spontaneous recanalisation of LAD with aneurysmal appearance (Figure 2A). The coronary dissection was not apparent angiographically. To understand this ambiguous appearance of LAD by angiography, we performed an intravascular ultrasound (IVUS). IVUS demonstrated a coronary artery dissection with a long and huge pseudoaneurysm (Figures 2B and 2C).
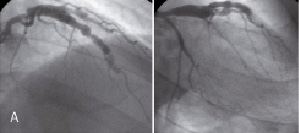
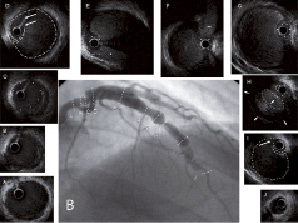
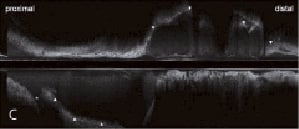
Figure 2. A) Control angiography five months after SCAD shows ambiguous aneurysmal formation extending from proximal to mid-portion of LAD and the proximal portion of D2. B) IVUS five months after SCAD demonstrates a dissection with intimal tears as the entry and the re-entry (Panels D and I, arrows). A large false lumen creates a pseudoaneurysm. The true lumen (dot circle) is severely compressed by the huge false lumen. The pseudoaneurysm contains mural thrombi (Panel H, arrowheads) resulting in the small luminal size of the false lumen (dotted line circle) at the site of narrowing on angiography. The false lumen proximal to the entry point is fully thrombosed (Panel C). Side branches of the D2 (*) and septal (**) branches originate from the compressed true lumen, except for the D1 which originates from the false lumen. No dissections are observed in the distal LAD (Panel J), LAD ostium (Panel B) and LM (Panel A). C) Longitudinal IVUS shows SCAD with the long and huge pseudoaneurysm (arrowheads). The peudoaneurysm is observed just distal to the ostium of the LCX (*).
How could I treat?
The Invited Experts’ opinion
Recognition and treatment of spontaneous coronary artery dissection (SCAD) represents a challenge to the interventional cardiologist. Most of the times, diagnosis is performed at the time of the angiography with the typical appearance of a coronary dissection with the presence of a thin longitudinal radiolucent line representing an intimal medial flap with flow in two or more separate lumens1. This angiographic pattern often occurs in young women, during pregnancy or in patients with connective or inflammatory disorders or even atherosclerosis. Prognosis after surviving the initial event is usually good as most of dissections tend to seal over time. Thus, stent implantation is usually indicated only in the event of ongoing ischaemia or flow compromise. In such cases, careful catheter manipulation and intravascular or optical coherence tomography guidance is mandatory2,3. Surgery is left for patients with multivessel SCAD or left main involvement.
Far less common and more challenging was the clinical presentation observed by Furuichi et al. The patient presented an anterior ST elevation myocardial infarction five months before and the angiography revealed a huge dissection and pseudoaneurysm involving a long segment of proximal with occlusion of the mid-segment of the left anterior descending artery (LAD). Important diagonal branches originated from the aneurysmatic area (true or false lumen). The initial conservative approach (with dual antiplatelet therapy and heparin) was decided in that scenario and five months later distal LAD appeared to be recanalised. Regarding the initial approach, the avoidance of thrombolytic therapy was probably the key to avoid further expansion of the pseudoaneurysm. Dual antiplatelet regimen in combination with heparin given during admission could have helped limiting the false lumen thrombosis and the reopening distal vessel.
During the current admission the vessel was imaged by intravascular ultrasound. Main findings included: the distal LAD was patent and free of disease; the second diagonal branch and the septal branch originated from the true lumen whereas first diagonal did from the false lumen, true lumen appeared compressed at some segments by thrombus protruding from the false lumen; and, finally, the left main, the circumflex artery and the ostial LAD were also free of disease. At that stage the patient was in stable clinical situation, however, the outcome of this anatomical condition was unpredictable. On echocardiography, anteroapical hypokinesis was observed with an estimated ejection fraction of 45%. In view of the long-term prognosis of this young patient (LAD territory may be still viable as no akinesis was observed on echo), I would indicate revascularisation. What is debatable is the type of revascularisation to be performed. Stenting, although feasible, should include a very long segment starting from the healthy distal LAD segment until the ostium of the LAD which was also healthy. Intravascular ultrasound guidance should be mandatory and applied during the procedure. Several risks should be taken into consideration: occlusion of the diagonal branch originating from the false lumen; acute and persistent stent malapposition leading to an unpredictable risk of thrombosis during lifetime; indefinite duration of dual antiplatelet therapy; risk of longitudinal extension of the rupture during the procedure (circumflex, left main,...) or vessel rupture (tamponade); risk of distal embolisation of thrombotic material. Balancing the risks and benefits of revascularisation, I would indicate off-pump surgical revascularisation with left internal mammary artery sequentially grafted into the diagonal branch and distal LAD.
How could I treat?
The Invited Experts’ opinion
The increasing use of primary PCI has probably resulted in an increased recognition of spontaneous coronary artery dissection (SCAD) as a cause for acute myocardial infarction. Optimal management of this case, five months after the index presentation, presents a number of challenges to the interventional cardiologist. There is marked aneurysmal coronary enlargement caused by dissected and dilated false lumens in both the LAD and the diagonals. The RCA is normal. When we consider potential therapies, we should remember that this is not an atheromatous coronary artery and that mechanisms of flow disturbance leading to ischaemic symptoms have little in common with the majority of patients that we treat daily with coronary atheroma. Indeed, one might consider that the disease process in SCAD has close parallels with aortic dissection and aneurysm formation. In patients with spontaneous aortic haematoma resolution of intramural haemorrhage (a pathophysiological feature often unrecognised in SCAD patients) can result in both acute dissection and aneurysm formation during follow-up1.
When SCAD patients present with acute infarction, bypass surgery is usually inappropriate. The vessel is often dissected extensively making a distal bypass difficult to achieve safely particularly in a haemodynamically unstable patient2. However in this case, it is now some months later and the distal LAD and diagonals are spared from dissection (confirmed by the IVUS). LAD and main diagonal are abnormal thus obtaining a lifetime result with percutaneous intervention is difficult. Consequently despite my lifetime personal bias, my management recommendation would be bypass surgery using the LIMA.
If intervention is to be undertaken, I would use IVUS to help differentiate anatomy and confirm wire position in the true lumen2. Minimising side branch occlusion should be a priority and this would preclude use of covered stents for most of the vessel. A provisional bifurcation stenting approach is the most suitable technique for the LAD with sequential kissing balloons to open restricted branch orifices. The risk of plaque shift should be low as the vessels are likely to be free of atheroma. Although I have previously used drug eluting stents (DES) in SCAD patients with small vessels3, in this case I would use bare metal stents as neointimal healing is likely to be beneficial in stabilising the arterial wall and preventing additional vessel dilation. Despite procedural IVUS usage there is a risk of incomplete apposition and one might speculate that there could be an enhanced risk of late acquired malapposition during follow-up. A reduced duration of dual antiplatelet therapy with bare metal stents may also be beneficial. In my opinion, DES could be used at a later date if symptomatic restenosis occurred.
Finally we should remember there is a risk of further spontaneous dissection in the remaining coronaries. This risk might be reduced by beta blockers and I would probably recommend this therapy life long. If there is evidence of type 6 Ehlers Danlos syndrome some genetic counselling may be appropriate.
How did I treat?
Actual treatment and management of the case
A conservative strategy was selected adding oral anticoagulant therapy to dual antiplatelet therapy, -blocker and ACE inhibitor. However, the patient developed reinfarction one month after hospital discharge. He received medical treatment in the local centre. One month after reinfarction, he came to our hospital for coronary angiography. Control angiography showed slow-flow in LAD (Figure 3). Thus we decided to treat LAD by PCI. Pre-intervention IVUS confirmed the placement of the guidewire in the true lumen and showed luminal narrowing of the false lumen due to further mural thrombi formation (Figure 3). Four Xience V stents (Abbott Vascular, Redwood City, CA, USA) were implanted in LAD and one in the second diagonal branch (D2) (Figure 4). The patient was discharged with dual antiplatelet therapy, -blocker and ACE inhibitor.
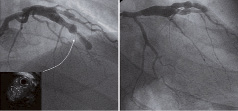
Figure 3. Control angiography one month after development of reinfarction reveals slow-flow in the LAD. IVUS shows the narrowing of the false lumen (dotted line circle) due to further mural thrombi formation.
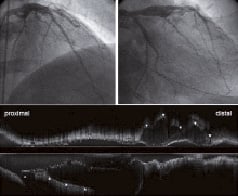
Figure 4. Coronary angiography and IVUS after implantation of everolimus-eluting stents in the LAD and D2 show the residual flow in the pseudoaneurysm only in the proximal portion and the focal mid-potion of the LAD where the entry and re-entry points were observed nearby. The asterisk on the longitudinal IVUS image indicates LCX.
At 4-month follow-up the pseudoaneurysm became smaller compared to pre- or post-intervention, however, the flow in the pseudoaneurysm in the proximal portion and the mid-portion of the LAD remained (Figure 5). The patient had been in stable condition for nine months after PCI. In particular, at 9-month follow-up angiography, the focal residual flow in the pseudoaneurysm in the mid-LAD disappeared (Figure 6).
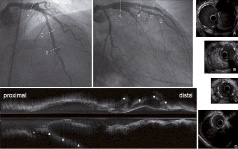
Figure 5. Four-month follow-up angiography and IVUS after PCI show residual flow in the pseudoaneurysm in the proximal portion (Panel A) and the focal mid-portion of LAD (Panel D). The other part of the pseudoaneurysm is completely thrombosed (Panels B and C). Angiography and longitudinal IVUS reveal smaller pseudoaneurysm compared to pre- or post-intervention evaluation.
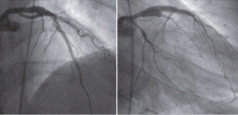
Figure 6. Nine-month follow-up angiography after PCI shows no residual flow in the pseudoaneurysm in mid LAD. The dissection flow in the proximal LAD has remained. The pseudoaneurysm is smaller in size compared to pre-intervention angiography.
Discussion and conclusion
SCAD is a rare condition characterised by a dissection plane which runs in the outer media or between the media and adventitia1. The clinical features are typically of an acute coronary syndrome or sudden death in a patient whose age and sex suggest a low probability of atherosclerotic disease. SCAD has been reported in association with multiple factors including the peripartum period, oral contraceptive use, Ehlers-Danlos syndrome, Marfan syndrome and cocaine use1. None of these were observed in our patient.
The natural history of SCAD is unpredictable. SCAD can heal spontaneously2,3, however, it can rarely create a pseudoaneurysm4-6. In addition, coronary pseudoaneurysms due to SCAD may be difficult to diagnose by angiography. There have been only three case reports of patients of pseudoaneurysms after SCAD: all pseudoaneurysms in these reports were focal. In contrast, to the best of our knowledge, this is the first report of a long diffuse coronary peudoaneurysm after SCAD treated by PCI. Pande et al reported a case of SCAD with aneurysmal formation in RCA detected by angiography4. The patient also had critical stenosis in the LAD and coronary artery bypass grafting was performed. The diagnosis of SCAD with a pseudoaneurysm was confirmed at the time of surgery. Aquel et al presented a case of SCAD with a pseudoaneurysm in the LAD diagnosed by IVUS5. A bare-metal stent was successfully implanted. Chabrot et al described a case of a postpartum female with a pseudoaneurysm in the LM six weeks after SCAD6. The diagnosis of a pseudoaneurysm was made by MDCT. The patient was successfully treated with implantation of paclitaxel-eluting stents. In our case, the coronary dissection was not apparent angiographically. In this setting, IVUS was quite useful to confirm the diagnosis on the ambiguous aneurysmal structure. Furthermore, optimal management of a pseudoaneurysm after SCAD is unclear. We firstly selected medical therapy adding oral anticoagulant therapy to dual antiplatelet therapy, β-blocker and ACE inhibitor. This appeared logical since platelet-rich thrombi might develop secondary to transient or sustained vessel occlusion from the dissection flap. However, one month later the patient developed reinfarction. Because of the very long lesion, we implanted everolimus-eluting stents to avoid restenosis. Stent implantation for SCAD can restore flow in the true lumen, seal the dissection plane and avoid further expansion of the false lumen. There have been few case reports on drug-eluting stent (DES) implantation for SCAD7,8. More data are needed to clarify feasibility and efficacy of DES implantation in patients with SCAD.
In conclusion, SCAD can cause a long diffuse coronary pseudoaneurysm which might be difficult to diagnose on angiography. IVUS can provide a definite diagnosis for ambiguous lesions by angiography. Moreover, IVUS can guide the operator to place a guidewire in the true lumen and seal the entire dissected segment with stents during PCI for SCAD.
Online data supplement
Moving image 1. Baseline coronary angiography showing a coronary dissection in the LM and the proximal LAD.
Moving image 2A. Control angiography five months after SCAD.
Moving image 2B. IVUS in the distal segment of the LAD.
Moving image 2C. IVUS in the proximal segment of the LAD and LM.
Moving image 3A. Control angiography one month after reinfarction.
Moving image 3B. IVUS one month after reinfarction.
Moving image 3C. Final angiography after PCI.
Moving image 3D. Post-intervention IVUS in the distal segment of LAD.
Moving image 3E. Post-intervention IVUS in the proximal segment of the LAD and LM.
Moving image 3F. Post-intervention IVUS in the D2.
Moving image 4A. Four-month follow-up angiography after PCI.
Moving image 4B. Four-month follow-up IVUS in the distal segment of the LAD.
Moving image 4C. Four-month follow-up IVUS in the proximal segment of the LAD and LM.
Moving image 5. Nine-month follow-up angiography after PCI.

