
Observations from contemporary spontaneous coronary artery dissection (SCAD) case series have led to expert consensus recommendations that conservative therapy is preferred and, in the absence of high-risk features such as ongoing ischaemia, left main dissection, electrical or haemodynamic instability, should be the first-line treatment strategy for SCAD1-3. This is based on the premise that the majority of SCAD arteries heal spontaneously, and that revascularisation is associated with high failure rates. Nevertheless, revascularisation is necessary in a minority of SCAD patients, and the optimal approach is controversial given the lack of randomised trial data. As such, long-term observational real-world data are the only available resources for clinicians to glean from for guidance with decision making on the best management strategy. In this issue of EuroIntervention, two such reports on long-term outcomes with conservative therapy and bioabsorbable stents help shed some light on the management of this challenging condition4,5.
The pathophysiology of myocardial infarction with SCAD is predominantly due to compression of the true lumen by intramural haematoma (IMH) in the false lumen, and sometimes by intimal dissection flaps (with or without overlying thrombus) that can encroach against the true lumen. Angiographic series have shown that most SCAD (>75%) have a type 2 (long diffuse narrowing) and/or type 3 appearance (focal/tubular stenosis mimicking atherosclerosis) due to IMH, without the presence of intimal dissection angiographically6. In the absence of instrumentation of the SCAD lesions with wiring or angioplasty/stenting, the natural history appears to be spontaneous healing with gradual resorption of the IMH and tacking-up of the intimal flap against deeper arterial layers. Several retrospective case series have reported that spontaneous angiographic healing occurred in 73-97% of cases when repeat angiography was performed1. In the largest series of 182 SCAD lesions with repeat angiography, 95% of lesions had healed when the angiograms were performed ≥30 days post SCAD.
There appeared to be time dependency for these processes of arterial healing to occur. On OCT, early resorption of IMH has been described as starting within days6. Full resorption will probably require weeks to occur, depending on the volume of IMH within the false lumen. Figure 1 shows an example of angiographic healing and a resolving IMH 17 days post SCAD on OCT. In the case report by Amabile et al4, the authors inadvertently wired into the false lumen and performed thrombectomy, but interestingly decreased the false lumen IMH and helped to decompress the true lumen. However, repeat angiography and OCT five years later showed a persistent false lumen due to instrumentation of the dissected artery, which interrupted the natural healing process and subsequent healed appearance, as exemplified in Figure 1.
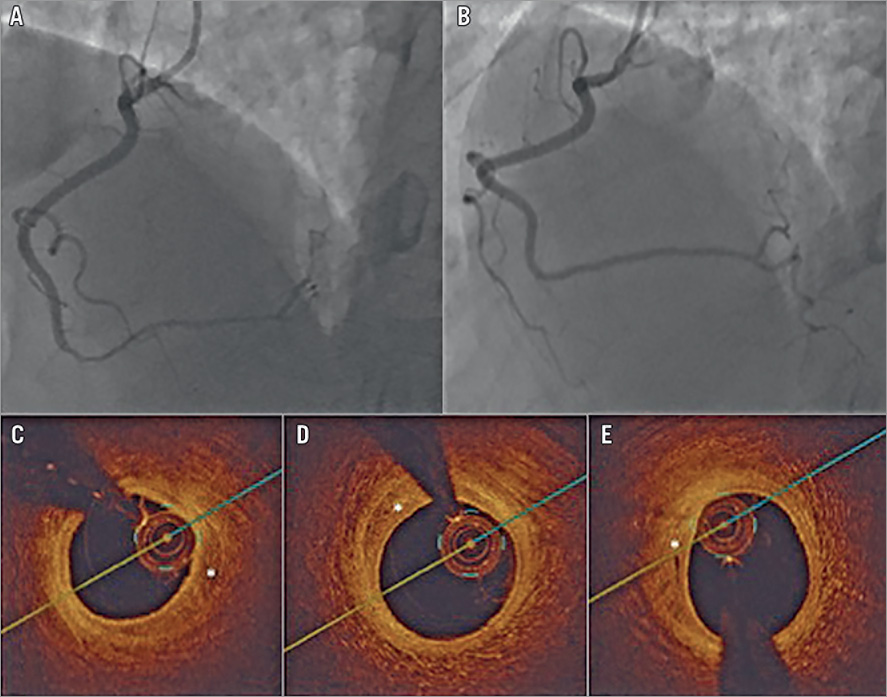
Figure 1. Example of angiographic healing and a resolving IMH 17 days post SCAD on OCT. A) Distal right coronary artery type 2 SCAD. B) Repeat angiogram 17 days later showing angiographic healing. C) - E) Optical coherence tomography images 17 days after SCAD showing resolving intramural haematoma (*).
In cases where revascularisation is necessary (e.g., clinical evidence of ongoing ischaemia, cardiogenic shock, sustained ventricular arrhythmias, left main dissection) and if percutaneous coronary intervention (PCI) is technically feasible, this is generally preferred over coronary artery bypass surgery (CABG), unless patients have left main dissection or failed PCI. However, PCI for SCAD can be technically challenging and is associated with high failure rates and risk of complications. Reported SCAD PCI success rates range from 47-91%1. The challenges related to SCAD PCI include increased risk of iatrogenic catheter-induced coronary artery dissection, difficulty wiring into the true lumen, and propagation of the dissection or IMH with wiring, angioplasty or stenting. If stenting is required, typically dissected segments are extensive, necessitating multiple long stents that can increase the risk of restenosis and stent thrombosis. Moreover, late stent malapposition can occur with resorption of IMH over time: Lempereur et al described the appearance of stent malapposition on OCT at various stages after SCAD PCI with IMH resorption7. These challenges should be taken into consideration when strategising SCAD PCI, including avoiding stent implantation if possible.
Given the high risk of iatrogenic dissection in SCAD patients (reported incidence of 3.4%: 2.0% with diagnostic angiography, and 14.3% with ad hoc PCI)8, meticulous care while manipulating catheters, engaging the coronary arteries, and performing contrast injections should be practised. The next step of wiring into the true lumen is also critical. This can be challenging especially in cases of type 1 SCAD where there is clear intimal disruption and a higher risk of wiring into the false lumen. Operators need to confirm that the coronary wire is in the distal true lumen before proceeding with angioplasty or stenting. Indeed, the PCI procedure should be aborted if the distal true lumen cannot be wired. Once the vessel is successfully wired, there are many different technical options for angioplasty and/or stenting. These include: 1) balloon angioplasty alone, 2) cutting balloon angioplasty to fenestrate the intima allowing decompression of the IMH in the false lumen, with or without additional stenting, 3) use of long stents to cover both edges of dissections by >5 mm to decrease the IMH propagation, 4) stenting the distal and proximal ends of the dissection with short stents before stenting the middle of the dissection to prevent IMH propagation, 5) focal stenting of the proximal segment of the dissection (or just proximal to the dissection) to prevent proximal IMH extension, and 6) use of bioabsorbable stents (if available) when long stents are required. A suggested PCI algorithm according to the angiographic SCAD subtypes is shown in Figure 2.
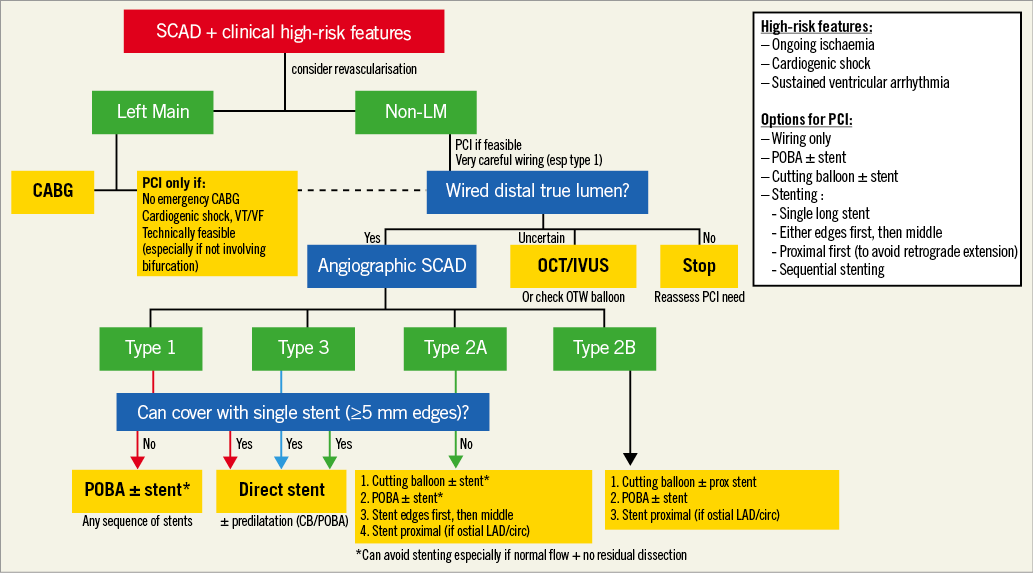
Figure 2. Suggested algorithm for SCAD PCI. CABG: coronary artery bypass graft; CB: cutting balloon; IVUS: intravascular ultrasound;
LAD: left anterior descending artery; LM: left main coronary artery; PCI: percutaneous coronary intervention; POBA: plain old balloon angioplasty; SCAD: spontaneous coronary artery dissection; VF: ventricular fibrillation; VT: ventricular tachycardia.
Of these approaches, two (cutting balloon and bioabsorbable stent) deserve further discussion since metal scaffolding may be avoided. The novel use of a cutting balloon to fenestrate the intima to allow decompression of the false lumen is highly appealing for SCAD treatment, since it improves true lumen flow and may circumvent the use of stents. To date, there are limited data on the use of cutting balloons for SCAD, with <20 patients in case reports and small case series9,10. Generally, cutting balloon angioplasty should be performed cautiously with small diameter balloons at least 0.5 mm smaller than the calibre of the vessel being intervened, and short balloons (6 or 10 mm length) given the tortuosity of dissected arteries. Low-pressure (up to 4 atm) inflations should be used to decrease the risk of coronary perforation. The goal of fenestration should be to restore TIMI 3 flow and alleviate ischaemia, which may require multiple balloon inflations. If cutting balloon angioplasty is insufficient to restore TIMI 3 flow and resolve ischaemia, then stents should be deployed10.
The use of bioabsorbable stents is theoretically appealing given the temporary scaffold, especially when long stents are required1. The study by Macaya et al is the largest SCAD series (n=22) treated by bioabsorbable stents5. The majority of the dissected arteries involved the left anterior descending artery (73%), and 36% involved proximal arteries (9% left main). The mean dissection length was 47.8 mm and mean reference vessel diameter was 2.9 mm. Intracoronary imaging was used in 86% of patients to optimise stenting. Extension (propagation) of the dissection occurred during the procedure or in-hospital in 22.7% with PCI. All patients were treated with dual antiplatelet therapy (DAPT) post PCI (median 12 months), with 55% receiving ticagrelor, 18% prasugrel, and 27% clopidogrel. At a median follow-up of 3.5 years, 78% underwent repeat angiography and there were two repeat revascularisations (one due to stent shrinkage, and one due to persistent dissection flap distal to the stented segment). There were no cases of stent thrombosis, restenosis, myocardial infarction or death. In a prior study by Ielasi et al, 18 SCAD patients were treated with bioabsorbable stents, with a mean diameter of 3.7 mm, a mean length of 24.1 mm, and overlapping stents required in 61.1%11. There were no clinical events at a median 18 months of follow-up.
In aggregate, both these studies suggest that bioabsorbable stents appeared safe for patients with SCAD, with no reported occurrence of stent thrombosis at longer-term follow-up. However, these studies are small and there is no comparative study to other PCI approaches for SCAD. Thus, the optimal approach with PCI for SCAD remains unclear. For example, is there an advantage of stenting versus angioplasty alone? Is there an advantage of cutting balloon angioplasty versus standard balloon angioplasty? Is there an advantage of metal versus bioabsorbable scaffold? These clinically relevant and important questions for the management of this challenging disease remain unanswered. Furthermore, the optimal antiplatelet regimen and duration post PCI for SCAD is unknown. DAPT appears reasonable for one year after drug-eluting stent placement, should probably be longer with bioabsorbable stents for SCAD patients, and can probably be shorter with angioplasty alone. Clopidogrel was most often used, but more potent agents such as ticagrelor or prasugrel should be considered, especially if there are residual dissection flaps (such as with cutting balloon usage without stenting).
There remain many unanswered questions on the management of SCAD patients. This being a relatively rare disease makes conducting large randomised trials unfeasible. Therefore, real-world experience is instrumental and will continue to be the main data source to guide our management of SCAD patients. Continued publication of case reports and series is highly encouraged to advance further our knowledge of this still poorly understood condition.
Conflict of interest statement
J. Saw has no conflicts of interest with regard to this editorial. She has received unrestricted research grant supports (from the Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, National Institutes of Health, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, Abbott Vascular, Boston Scientific, and Sunovion), consultancy and advisory board honoraria (AstraZeneca, Boston Scientific, and Abbott Vascular), and proctorship honoraria (Abbott Vascular and Boston Scientific).

