
CASE SUMMARY
BACKGROUND: A 44-year-old male, with a history of primary PCI on the LAD, presented with high-risk unstable angina and was found to have a sub-occluded RCA. During PCI on the RCA an extensive dissection of the proximal RCA, aortic sinus of Valsalva, aortic arch and descending aorta occurred.
INVESTIGATION: Coronary angiography, laboratory test, electrocardiography, transthoracic echocardiography, computed tomography, transoesophageal ultrasound.
DIAGNOSIS: Iatrogenic dissection from the proximal RCA to the descending aorta.
MANAGEMENT: Bare metal stenting, followed by special graft stenting of the RCA ostium, medical therapy during PCI, hospitalisation and follow-up.
KEYWORDS: aortic dissection, coronary angioplasty, stent graft
PRESENTATION OF THE CASE
With an incidence of less than 0.1%, aortic dissection is a potentially catastrophic complication during coronary artery catheterisation1, limited to the ascending aorta in most cases2,3. We report a very rare case of iatrogenic type IIA aortic dissection during percutaneous coronary intervention (PCI) of the right coronary artery (RCA).
A 44-year-old hypertensive male (stomatologist), with a history of anterior myocardial infarction (MI) and primary PCI (10 months prior), an active smoker (20 packs per year) was referred to our catheterisation laboratory for coronary angiography with high-risk unstable angina. He was an overweight male with a BMI of 33.2 kg/m2, with an important family history of coronary artery disease (CAD) (father having had an MI and aortocoronary bypass), on medication (aspirin, clopidogrel, beta-blocker, ACE inhibitor and statin). The electrocardiogram (ECG) showed sinus rhythm, slow transition of R-wave in the anterior leads, and 1 mm ST depression in the inferior leads. Echocardiography showed a 35-40% left ventricle ejection fraction (LVEF) with severe hypokinesia of the anterior wall and mild hypokinesia of the posterior/inferior wall. The troponin I (TnI) was negative for MI.
Left coronary system injection showed a mild and non-significant in-stent restenosis in the proximal left anterior descending (LAD) coronary artery (Figure 1), with no significant lesion elsewhere; RCA injection showed a subtotal occlusion in the mid-proximal segment, with distal blood flow of Thrombolysis In Myocardial Infarction (TIMI) grade 2-3 (Figure 2). Given the patient’s history, the ECG changes and the ongoing symptoms on maximum medical therapy, a decision was made to proceed with PCI to the RCA. Unfractionated heparin (UFH) had been given with a dose of 80 IU/kg.
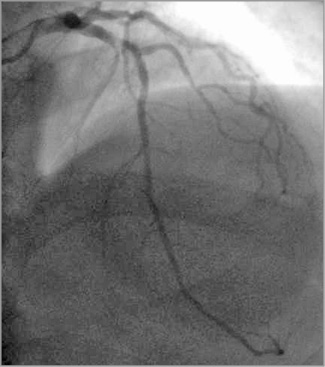
Figure 1. LAD: mild and non-significant in-stent restenosis in the proximal segment of LAD.
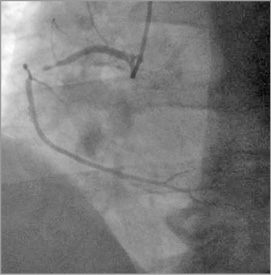
Figure 2. RCA: subtotal occlusion in the mid-proximal segment of the RCA, with distal blood flow of Thrombolysis In Myocardial Infarction (TIMI) grade 2-3.
The RCA ostium was engaged with a 6 Fr Judkins right 4 (JR4) guiding catheter (Launcher® Coronary Guide catheter; Medtronic, Minneapolis, MN, USA), via the right radial artery using a 6 Fr arterial sheath. The sub-occlusion in the mid-proximal RCA was easily crossed using a 0.014” PT2™ guidewire (Boston Scientific, Marlborough, MA, USA). A bare metal stent (BMS), CoroFlex® Blue Neo, 3.0×19 mm (B. Braun Melsungen AG, Berlin, Germany)/14 atm was directly implanted in the mid-proximal RCA.
A coronary spiral dissection was noted proximal to the stent and back to the right coronary sinus and ascending aorta, most likely induced by stenting rather than the guiding catheter. An important spasm in the distal part of the stent was also noted (Figure 3) with TIMI 1 distal flow. Abrupt onset of severe chest pain and ST-segment elevation in the inferior leads followed by hypotension occurred. The aortic dissection had extended to the ascending aorta and aortic arch, but the great vessels of the neck appeared not to be involved.
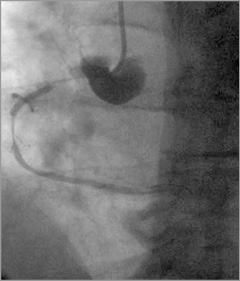
Figure 3. RCA: immediately after BMS (3.0×19 mm) implantation, severe spasm distal to the stent and dye staining in the aorta right coronary sinus.
A second BMS CoroFlex Blue Neo, 3.5×32 mm (B. Braun Melsungen AG)/14 atm was implanted from the first BMS (overlapped 3 mm) to the ostium of the RCA (3 mm out of the RCA) (Figure 4). The second BMS restored the distal RCA flow (TIMI 2-3), but did not seal the entry site of the aortic dissection (apparently paraostial, but covered by the second BMS) (Figure 5), and an extension to the descending aorta occurred during injections (Figure 6). The chest pain was still high, but there was no more hypotension. At that moment, was any other percutaneous solution available or should the patient be sent urgently for surgery?
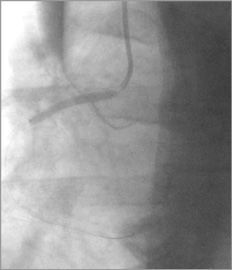
Figure 4. RCA: second BMS (3.5×32 mm) implantation from the first BMS (overlapped 3 mm), to the ostium of the RCA (3 mm out of the RCA).
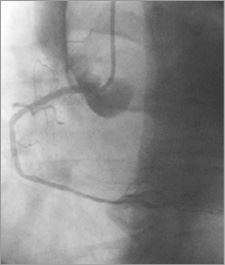
Figure 5. RCA: the distal RCA flow - restored (TIMI 2-3); entry site of the aortic dissection still open.
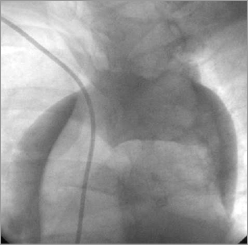
Figure 6. Aorta: extension of dissection to the descending aorta.
How would I treat?
THE INVITED EXPERTS’ OPINION

Due to the rare occurrence of iatrogenic aortic dissection (IAD) as a complication of percutaneous coronary intervention, the available information is limited and management of this complication has not been very well defined. However, most cases can be managed either conservatively, or with immediate stenting of the coronary ostium, to “seal off” the entry point of the false lumen. Covered stents can also be used similarly to non-covered conventional stents4. As with all ostial stenting procedures, achieving full ostial coverage with minimal stent protrusion is essential. The high fibrous content of the coronary ostium may require higher pressures to achieve complete expansion of the stent. Although accurate positioning should be confirmed in multiple projections, forceful contrast injections into the false lumen can result in further propagation of the dissection, highlighting the need to limit contrast injections. Alternatively, angiographic landmarks and contrast staining of the sinus of Valsalva can be used, although they can be misleading. Intravascular ultrasound (IVUS) can be useful in defining the true position of the ostium for accurate stent positioning. If the dissection is not extensive and does not seem to evolve, the patient will probably do well with an interventional approach. Indeed, in a recently published series of 74 patients with a follow-up for a median of ~51 months, the authors suggested excellent spontaneous resolution of this complication after the acute phase5.
There is controversy over which patients should be referred to surgery. The decision usually depends on the haemodynamic status of the patient, development of aortic regurgitation or pericardial effusion/tamponade, extension of the dissection and involvement of the great vessels of the aortic arch. Whilst Dunning et al recommended surgery for dissections involving the cusp and extending up the aorta more than 40 mm, which they called class III dissections1, successful interventional management of these patients has also been reported6. Additionally, development of IAD in the context of acute coronary syndromes makes the decision for surgery more complicated since these patients are often receiving vigorous antithrombotic and anticoagulant treatment.
In this particular case, the patient appears to be haemodynamically stable without any involvement of the aortic arch vessels. At this point, we would implant an additional stent in the ostium, preferably utilising IVUS to ensure ostial coverage. After that, we would perform transoesophageal echocardiography (TEE) while the patient was still on the table. In addition to showing the intimal dissection flaps, true/false lumens, and giving a better understanding of the extension of the dissection, TEE can further reveal the presence and severity of aortic regurgitation. Given that the dissection progression is limited, signifying a stable state, close observation of this patient may be sufficient, despite the involvement of the ascending aorta. Additional medical therapy may be administered to achieve pain relief and blood pressure control, titrating around the low normal systolic pressure targets, using morphine sulphate, beta-blockers and IV vasodilators. An emergent referral for surgical repair may be warranted if haemodynamic instability develops, or imaging reveals extension of the disease, particularly to the great vessels in the aortic arch. Provided that the patient remains stable, additional TEE, computed tomography or magnetic resonance imaging may be carried out prior to discharge for future reference.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

This is an unfortunate case of iatrogenic retrograde type A aortic dissection (AD) after coronary artery stenting of the right coronary artery (RCA) in a 44-year-old patient.
With the increase in complex interventions including percutaneous coronary intervention (PCI), thoracic endovascular aortic repair (TEVAR), and transcatheter aortic valve replacement (TAVR), there has been an increase in iatrogenic aortic dissection5. There are two main types of AD during coronary intervention: a retrograde form, which usually seals alone without further treatment, and an antegrade form usually with an entry point inside a coronary artery which can be sealed with a stent. Many of the reported cases of right coronary artery dissection extending into the aorta are likely to be secondary to catheter manipulation and injection with a deep catheter engagement in the ostium of the coronary artery.
Dunning et al1 previously categorised AD due to coronary interventions into three grades: type 1 was an aortic dissection limited to the sinus of Valsalva, a type 2 dissection involved the ascending aorta but was <4 cm, and a type 3 dissection exceeded 4 cm in length. Stenting was usually sufficient to solve the dissection when a coronary artery was involved, but surgery was warranted in a type 3 dissection.
This is how we would have treated this case step by step. The operators first noted a coronary spiral dissection proximal to the mid-RCA stent extending to the right coronary sinus and ascending aorta. After identification of a coronary dissection with extension into the aorta (grade 1, 2), it is crucial not to lose wire position and essential to stop dye injections as this will further extend the dissection caused by hydraulic pressure. We would then have proceeded with stenting of the mid-RCA with an overlapping stent extending 2 mm beyond the ostium of the RCA in order to seal completely the entry point of the dissection. Then, we would have used intravascular ultrasound to ensure adequate deployment of the stent and full apposition at the ostium of the RCA avoiding dye injections. With this approach, the dissection entry point is sealed and a conservative strategy can be undertaken with a watch and wait approach.
Unfortunately, in this case, the AD extended into the descending aorta after deployment of the second BMS. At this point, the case was transformed from a somewhat controlled situation to a more complex and potentially life-threatening one.
In general, patients with acute dissection involving the ascending aorta (type A) should be considered for emergency cardiac surgery, especially in younger patients (<70 years). However, the very nature of this iatrogenic problem may allow a simpler and rational solution - cover up the coronary problem, “wait and watch” at close intervals using CT to ascertain whether the dissected aortic wall segment will readapt and heal7,8. Such a conservative strategy, however, is only feasible and permissible if there is no evidence of extravascular bleeding, pericardial tamponade, or organ malperfusion. The second step is to maintain the patient under close surveillance, lower blood pressure in an intensive care unit, and perform repetitive imaging at short intervals. Emergency surgical repair will be recommended for any life-threatening extension (evaluated by imaging) of the dissection.
Alternatively, a percutaneous approach with TEVAR could be proposed. The goals of TEVAR are similar to surgical intervention, i.e., to cover the origin of the intimal tear in order to prevent aortic rupture as well as to reduce pressure and promote thrombosis of the false lumen and prevent aortic branch malperfusion9. Two options are possible.
– A polytetrafluoroethylene (PTFE)-covered stent graft, deployed retrogradely from a femoral approach, is positioned with the proximal landing zone at the sinotubular junction and the distal landing zone proximal to the brachiocephalic trunk. This will seal the entry point with the possibility of remodelling the dissected ascending aorta. A similar successful strategy was described by Conzelmann et al10 in iatrogenic AD after TAVR with complete sealing of the entry point.
– An uncovered stent may be used and implanted from the ascending aorta to the descending aorta across the aortic arch. This might not seal the dissection completely but will scaffold and support the flap throughout its length to achieve false lumen stabilisation, depressurisation, remodelling, and eventual false lumen thrombosis. This approach will allow full perfusion of the neck vessels and cause distal false lumen thrombosis. A similar successful strategy was described by D’Ancona et al11 after iatrogenic AD during TAVR.
In summary, it appears reasonable to adhere to a strategy of watchful waiting with surgery or TEVAR in case the patient develops symptoms or signs of progressive aortic dissection.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
A third stent, but this time a stent graft GRAFTMASTER® RX, 3.5×19 mm (Abbott Vascular, Santa Clara, CA, USA)/16 atm, was implanted from the RCA ostium, sealing the entry site of the aortic dissection (Figure 7). After successful coronary graft stenting, TIMI 3 flow was achieved, persistent dye staining of the aorta diminished (Figure 8-Figure 10), and the patient’s condition stabilised. The ECG and haemodynamic changes were quickly back to normal.
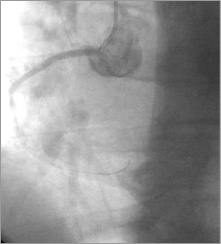
Figure 7. RCA: image after stent graft 3.5×19 mm implantation from the RCA ostium, sealing the entry site of the aortic dissection.
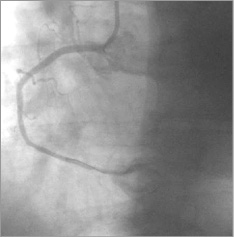
Figure 8. RCA: distal blood flow of TIMI grade 3.
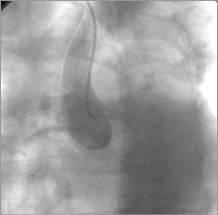
Figure 9. Aorta: dye staining in the ascending aorta.
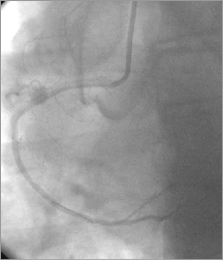
Figure 10. RCA/aorta: persistent dye staining of the aorta diminished.
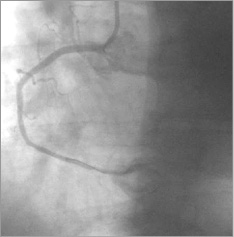
Figure 8. RCA: distal blood flow of TIMI grade 3.
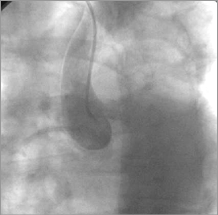
Figure 9. Aorta: dye staining in the ascending aorta.
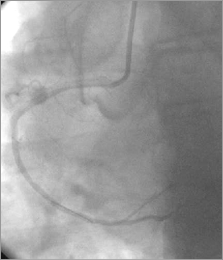
Figure 10. RCA/aorta: persistent dye staining of the aorta diminished.
Computed tomography (CT) angiography was performed immediately after the procedure. A type IIA DeBakey-Stanford aortic dissection, originating from the RCA ostium and extending to the descending aorta, up to T5 (thoracic segment – T5 vertebra delimited) was noticed (Figure 11, Figure 12). There was no involvement of the great neck vessels and a maximum of 13 mm diameter in the false lumen, but without evidence of an intimal flap.
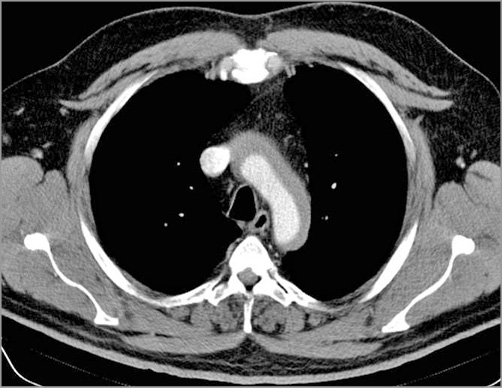
Figure 11. Computed tomography (CT) angiography: dissection of the aortic arch.
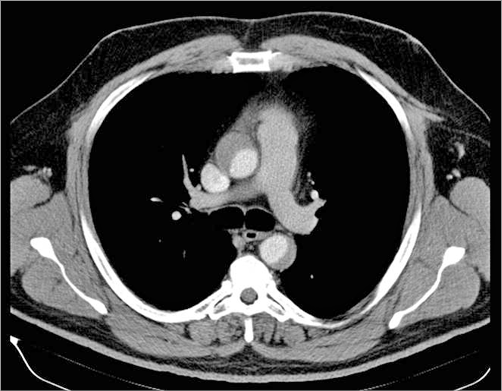
Figure 12. CT: dissection of the ascending and descending aorta, up to T5 (thoracic segment – T5 vertebra delimited).
Every day, transthoracic echocardiography (TTE) was performed and revealed no abnormality (no aortic regurgitation or pericardial effusion) with LVEF 35-40%. Four days after PCI, on the day of discharge, transoesophageal ultrasound (TEE) was carried out which demonstrated significant reduction in the diameter of the false lumen (accepting the limitation between the CT and TEE measurements). The patient’s hospital course was uneventful. He was discharged on dual antiplatelet therapy (DAPT) –clopidogrel and aspirin (75 mg each daily)– for a minimum of six months.
A follow-up CT scan at six months showed complete resolution of the dissection, with full healing of the aorta and absorption of the haematoma. During all this time, the patient was asymptomatic and we extended the DAPT indication up to one year. He was scheduled for coronary angiography at nine months after the index event.
Discussion
Iatrogenic aortic dissection is a rare and potentially catastrophic complication of coronary artery catheterisation. In most cases, the entry site is paraostial of the RCA1-3. There are some morphological and structural differences between RCA and left main stem (LMS) aortic insertion as well as between the right and left aortic sinus, which may explain why the left aortic sinus is more resistant and therefore less liable to suffer due to iatrogenic dissection. Probably the diameter of the LMS (which is larger compared with the RCA), the acute angle between the LMS and the ascending aorta (compared to a nearly right angle for the RCA), and possibly the higher number of smooth muscle cells and collagen type 1 fibres covering the ostium segment and insertion of the LMS and left sinotubular junction, may explain the higher incidence of iatrogenic aortic dissection with an RCA entry site12. Most of these remain limited to the right coronary sinus and to the ascending aorta, these data being confirmed by angiography which may underestimate the true dissection extent, or by CT with highly accurate images of the dissection origin, extent and flap1,2,13. The case presented is very rare, because the aortic dissection was up to the descending thoracic aorta. Given that the most common scenario is catheter-induced dissection1-3, the particularity of this case was that the dissection had extended from the proximal part of the stent, 26 mm (quantitative coronary analysis [QCA]) backwards to the RCA ostium and right aortic sinus. This patient was hypertensive but with no evidence of aortic root dilatation or family history of Marfan syndrome or other causes of medial necrosis and therefore no histopathological specimen had been taken. He had had an acute MI prior to this event and he had presented at the index event with ACS. There was no variant anatomy of the coronary ostium, and no vigorous hand injection of dye or vigorous inspiration during dye injection had been noticed. These are the most common risk factors for iatrogenic aortic dissection1,14-16.
Due to the lack of numbers of cases reported, the optimal management of iatrogenic aortic dissection remains controversial. Probably most of them can be managed percutaneously followed by routine coronary care unit (CCU) observation. Even when the dissection is extensive, stenting the entry site in the dissection may be enough to stop it from expanding. If there are other complications, such as rapid progression of aortic dissection with involvement of the arch vessels, aortic regurgitation, pericardial effusion, or tamponade, then urgent surgical intervention is mandatory1-3,17.
There are two important particularities of this case: first, a bare metal stent did not seal the dissection entry site and it was necessary to use a stent graft. As far as we know18-22, this is the first case report of such an intervention. Second, the progression of the aortic dissection was up to the descending aorta, up to the T5 segment. It was uncertain whether surgery would have been necessary. Because the patient’s condition stabilised during the intervention, and he was asymptomatic, we decided, as a Heart Team, to keep him under observation in the CCU. This case also illustrates how, in the follow-up period, TEE and CT can show progression or regression of aortic and heart disease in a non-invasive manner.
Conflict of interest statement
The authors have no conflicts of interest to declare.

