Case summary
Background: A 61-year-old woman presented with one week of shortness of breath and was found to have occluded proximal right coronary artery with left to right collaterals. After predilation optical coherence tomography (OCT) imaging and power contrast injection was done to guide stenting therapy.
Investigation: Diagnostic angiography with bilateral injection of contrast injection, OCT, intravascular ultrasound of the aorta and MSCT.
Diagnosis: Iatrogenic right coronary artery and aortic dissection.
Treatment: Stenting of the right coronary artery and its ostium with covered stent and drug eluting stents; imaging with OCT post stenting; imaging of the aorta with peripheral IVUS and MSCT.
Keywords: Iatrogenic aortic dissection, total coronary occlusion, optical coherence tomography
Presentation of the case
A 61-year-old woman with coronary risk factors of hypercholesterolaemia, hypertension, mild renal insufficiency, smoking and past history of transient ischaemic events, presented with one week history of exertional shortness of breath. The ECG showed inferolateral ST depressions and troponin was positive. Aspirin, plavix and fraxiparine were administered. The patient underwent a diagnostic cardiac catheterisation which revealed a total occlusion of the proximal right coronary with left to right collateral. The patient was referred to this hospital for further percutaneous management of her single vessel disease.
Bilateral femoral access was obtained and dual catheter injections were used to visualise the collateral filling of the distal right coronary artery. An Amplatz Left-2 guide catheter was used to intubate the right coronary ostium and provided excellent support, although intubation caused pressure dampening. A left Judkins guide was used for left main injections. It became apparent that the RCA filled retrograde almost all the way up to the ostium and that the occlusion was relatively short (less than 15 mm). A HI-TORQUE PILOT 50 wire with over the wire 1.5×15 mm Ryujin balloon was used to cross the lesion (Figure1). After the initial crossing and balloon dilation forward flow was re-established. We intended to size the vessel prior to further balloon dilation and stenting with optical coherence tomography (OCT). An OCT wire was introduced into the distal RCA without difficulty. Pullback with a Lightlabs M3 system at 3mm/sec was done with power injection at 300psi of pressure at 3ml/sec. This resulted in aright coronary artery ostium dissection that propagated 8cm up the ascending aorta (Figures 2 and 3). Despite the extent of the dissection flow was maintained and the patient remained stable without hypotension, ST elevations or severe chest pain.
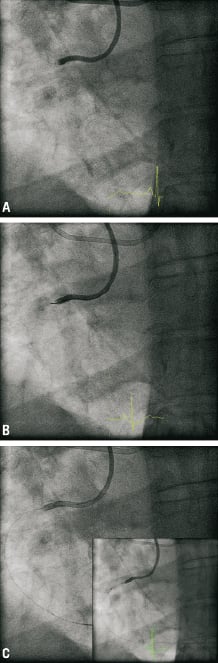
Figure 1. Angiogram: A) pre-procedure; B) PILOT 50™ 190 cm wire; C)after predilatation Ryujin plus™ OTW 1.5×15 mm balloon.
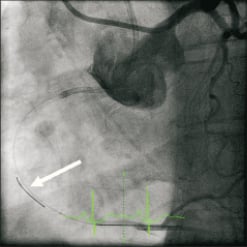
Figure 2. OCT. Lightlab Imagewire™ 0,019 inch no occlusion balloon. Flushmedium: Visipaque™ contrast Standard pump setting: 3 cc/sec, 300 PSI.
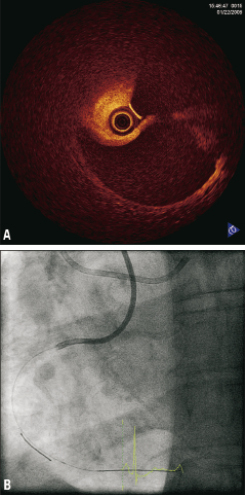
Figure 3. OCT. A) 3mm/sec pullback; B) Contrastflush 3cc/sec 300 psi.
How could I treat?
The Invited Experts’ opinion
This case describes a not so uncommon complication seen with rigid instrumentation of the right coronary ostium resulting in ostial dissection eventually propagating up the ascending aorta. By definition such scenario fulfils the criteria of a limited type A aortic dissection1,2; fortunately, there was no haemodynamic compromise with RCA occlusion from the dissected segment allowing to analyse and solve this case in no chaos and in structured fashion3,4. The first step according to my pathophysiologic understanding and experience should be to keep the coronary wire in place and finish the coronary intervention with the placement of a flexible intracoronary stent (preferentially a bare metal stent) to fix both the balloon-dilated proximal RCA lesion and simultaneously the ostial dissection. Care should be taken not to oversize the stent and to reach to the very ostium of the RCA and cover the intracoronary dissection up to the lumen of the aorta. In this way not only the initial goal e.g., to treat the RCA stenosis would be met, but also apotential entry point to the localised dissection of the ascending aorta would be closed raising the chances for sealing and healing of the entire dissected ascending aorta. Although this dissection is literally proximal (or type A) and traditionally requires surgery, the very nature of this iatrogenic problem may allow a simpler and logical solution: patch up the coronary problem, “wait and watch” in close intervals (by CT or TEE) if the dissected aortic wall segment will readapt and heal (as it often does)5-7. Such conservative “wait and watch” strategy, however, is only feasible and justifiable if there was no evidence of extra-aortic blood collection, pericardial effusion or evolving tamponade. The second step is to keep the patient under close surveillance, titrate blood pressure to low normal values preferentially on a surgical ICU and perform repetitive ultrasound imaging in short intervals. In summary, there is pathophysiologic background for the observational evidence that iatrogenic coronary artery dissection extending into the entire ascending aorta can be managed by successful stenting of the right coronary ostium; ultrasound or CT imaging may document complete resolution of aortic dissection8.
Conflict of interest statement
The authors have no conflict of interests to declare.
How could I treat?
The Invited Experts’ opinion
The right coronary artery (RCA) ostium presents a dissection, well identified by OCT, which propagated up to the ascending aorta (dense staining of the aortic wall with incomplete dye clearance after injection of contrast’ media). According to the classification proposed by Dunning et al1 it is a class III dissection (extension in the aorta greater than 40mm). It is an uncommon but impressive, and potentially a life-threatening complication, probably related to the deep engagement of the tip of the Amplatz guiding catheter.
In our experience at the Clinique Pasteur in Toulouse between 1999-2002, of a total of 7,359 percutaneous coronary intervention (PCI) procedures, we identified four cases (0.05%), all during PCI for RCA lesions, one class I (limited to the sinus of Valsalva, one class II (extension in the aorta less than 40 mm, and 2 class III) (J. Marco, invited lecture, ACC meeting 2002). These four cases were successfully stabilised without sequela by a stent implantation covering the ostium of the RCA followed by conservative management. Based on this experience and other reported experiences2-8, we would manage this complication by sealing the entry site with astent implantation followed by a conservative management and transoephageal echography (TOE) controls.
The most crucial technical points are to withdraw the tip of the guiding catheter within the aorta, to maintain the guidewire in place in the coronary lumen and to accurately place the proximal part of the stent within the aorta, while avoiding large amounts of contrast media injections within the coronary ostium that may aggravate the retrograde extension of the dissection.
We would place a 20×4.0mm stent with 3mm of the proximal part (2 struts) protruding within the aorta before the deployment. This accurate placement of the proximal part of the stent within the aorta is crucial and requires several controls in multiple projections without engagement of the tip of the guiding within the coronary ostium. The deployment of the stent should only be performed after assuring this condition: at least 3mm of the stent protruding within the aorta, which means that after the balloon inflation (up to 12-14 atmospheres) the proximal part of the stent will correctly covered the ostium of the RCA. After the stent deployment, a control by OCT or IVUS will be performed in order to be sure that the stent correctly cover the RCA ostium entry site as well as the distal part of the dissection and is correctly expanded. Then, at the end of the procedure, on the table, we will ask for a TOE control, which is the most appropriate non-invasive method to evaluate the length, extent and severity of the intramural haematoma in the aortic arch. This first TOE imaging will be the first reference allowing us to control the evolution.
Then the patient will be managed conservatively (ECG, clopidogel, aspirin, other medication and enzymes controls as for routine PCI).
A new control by TOE will be performed on day three in order to evaluate the stabilisation or potential evolution of the intramural haematoma (other option may be to control by MRI). If the intramural haematoma remains stable and the dissection sealed, the patient may be discharged with a new TOE (or MRI) planned after one month of follow-up.
In our experience and the majority of reported cases, sealing the entry site with a stent followed by a conservative approach was sufficient to resolve such complication without sequela.
Emergency surgical repair will only be proposed for life threatening evolutive extension (evaluated by sequential TOE or MRI) of the intramural haematoma and dissection to the horizontal arch and cranial branches uncontrolled despite rest and medically controlled low blood pressure. The risk of such intervention is very high (32%)4,6.
Conflict of interest statement
The authors have no conflict of interests to declare.
How did I treat?
Actual treatment and management of the case
The dissection likely involved only the intima. Given the patient’s stable vital signs and ECG we performed systematic distal to proximal stenting of the entire right coronary artery with drug-eluting stents (XienceV: 2.75×28mm, 2.75×28mm and 3.0×28mm in an overlapping fashion). Stenting of the ostrium was done with a Jomed Graftmaster covered stent (3.0×16mm) and drug eluting stent (XienceV 3.0×28mm) sandwich. This seemed to have sealed the dissection at the ostium and prevented further propagation of the aortic dissection. OCT was again performed showing very minimal dissection flap behind the stent struts and good stent apposition (Figure4). Final angiography showed a good result. The aorta was imaged using peripheral intravascular ultrasound (9MHz). This showed self-limited intimal dissection with some contrast in the false lumen (Figure5). The patient was transferred to the intensive coronary care unit in a stable condition for close blood pressure monitoring and control. MSCT was obtained a day later to confirm resolution of the dissection (Figure6). The patient was discharged to home in stable condition with the plan for repeat MSCT and cardiac catheterisation in six to eight weeks.
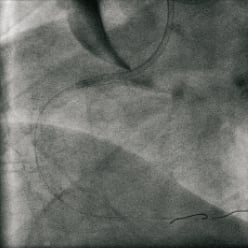
Figure 4. Result after 3x XienceV™ stents: Distal to proximal: 2.75×28mm, 3.0×28mm, 3.0×28mm; Proximal RCA: JoMed Covered stent, 3.0×16mm.
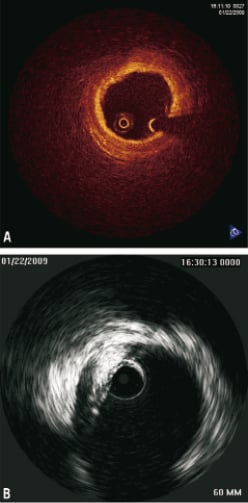
Figure 5. A) Final OCT: 3mm/sec flush: 3cc/sec 300 PSI; B) IVUS Aorta Ascendens: Volcano Visions™ 8.2Mhz IVUS catheter manual pullback
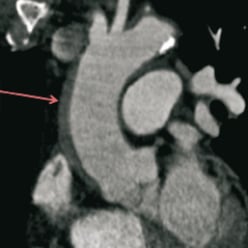
Figure 6. MSCT –one day post-procedure showing a stable intramural haematoma.
Discussion
Most of the iatrogenic dissection in the IRAD registry were caused by retrograde propagation of coronary dissection1,2. Patients who suffered this complication tended to be older, have a history of hypertension, coronary artery disease, and prior coronary artery bypass grafting. The diagnosis, although obvious in this case, may sometimes be challenging because, unlike spontaneous dissection, chest and back pain are often absent and less abrupt. Iatrogenic dissections, however, carry a mortality as high as that of spontaneous dissections (up to 35%) and have a higher incidence of concomitant myocardial infarction2,5.
Many of the reported cases of right coronary artery dissection extending into the aorta5-7 are likely secondary to catheter manipulation and injection with deep catheter engagement. This underscores the need for continuous monitoring of catheter tip pressures. Aortocoronary dissections have been amenable to treatment with ostial stenting of the right coronary and left main ostium8. In a published case series3 dissections extending less than 4cm above the coronary sinuses could be treated percutaneously and medically. Dissections involving more than 4cm of the ascending aorta usually require surgical management. In our case based the IVUS measurements performed intra-operatively and MSCT obtained the day after the procedure dissection flap was present at the level of the sinuses of Valsalva, however an intramural hematoma extended 7-8cm up the ascending aorta but did not involve any of the great vessels.
We chose to stent the ostium of the right coronary artery with asandwich of covered stent graft and drug eluting stent9.
Our case also serves as a cautionary tale as the OCT imaging becomes more prevalent in our day to day practice. Likely hand injection with careful attention to pressure rather than a power injection should have been performed and would have been sufficient to fill the vessel lumen and obtain adequate OCT imaging to size the vessel prior to stenting.
Conflict of interest statement
The authors have no conflict of interests to declare.

