case summary
Background: A 65-year-old woman with recent-onset angina and a positive stress test was admitted for coronary angiography. Risk factors were hypertension, hypercholesterolaemia and obesity.
Investigation: Planned percutaneous coronary intervention (PCI).
Diagnosis: Right coronary artery (RCA) dissection extending to the aortic root occurred during PCI.
Treatment: The entry site was closed with a coronary covered stent implanted into the RCA ostium. Multidetector computed tomography (MDCT) showed alarge aortic haematoma involving the aortic root and ascending aorta. Clinical tolerance was very good.
Keywords: coronary angioplasty complication management, intramural hematoma in the ascending aorta, coronary covered stent
Presentation of the case
A coronary angiogram performed three years earlier for non-ST-segment myocardial infarction had shown a right coronary artery (RCA) stenosis treated with implantation of two bare metal stents, as well as two intermediate lesions on the middle left anterior descending artery and obtuse marginal artery, respectively. Coronary angiography showed an in-stent total occlusion of the mid-portion of the RCA and no changes in the left coronary arteries (Figure 1 A). An ad hoc percutaneous coronary intervention (PCI) on the RCA was performed. A 6Fr guiding catheter (Amplatz Left 1.0; Cordis, Johnson & Johnson, Warren, NJ, USA) was inserted to improve stability, with no technical difficulties. Intubation was selective in the absence of a pressure drop, a guidewire (Asahi MEDIUM®; Abbott Laboratories, Abbott Park, Il, USA) was used to cross the lesion. Dissection of the RCA ostium extending to the aortic root and ascending aorta occurred, causing proximal RCA occlusion (Figure1B). A bare-metal stent (2.75mm-18mm, Micro-Driver® Rapid Exchange Coronary Stent System; Medtronic, Minneapolis, MN, USA) was implanted to close the entry site injury. Angiography then showed persistent extra luminal opacity, and a covered stent (3.0 mm-12 mm, JOSTENT GRAFTMASTER Coronary Stent, Abbott Laboratories) was implanted, which restored blood flow (Figure 1, C and D). Transthoracic and transesophageal echocardiography performed in the catheterisation laboratory showed no pericardial effusion or aortic regurgitation but visualised an intramural haematoma in the ascending aorta.
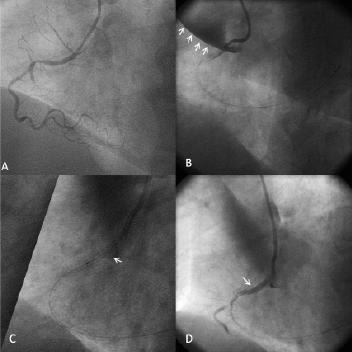
Figure 1. Coronary angiography. A: A 40° left oblique view showing total occlusion of the right coronary artery (RCA) at the second segment. B:spontaneous contrast medium leakage into the ascending aorta and pericardium. Dissection and occlusion of the first RCA segment. C:Coronary dissection treated by a covered stent. D: Final result. Anterograde progression of the contrast medium.
ECG-gated multidetector computed tomography (MDCT) performed one hour after angioplasty (Figure 2, A and B) showed that the intramural haematoma was 19 mm thick and involved the RCA ostium. On the non-enhanced MDCT images, high-density contrast medium was seen within the aortic wall and pericardial recess, extending to the origin of the right brachiocephalic artery. How should we treat this huge intramural haematoma: surgical management or conservative strategy with MDCT follow-up?
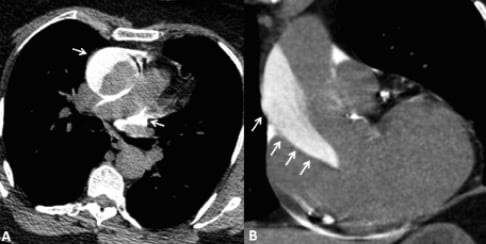
Figure 2. Non-enhanced multidetector computed tomography (MDCT) images of the ascending aorta in the axial (A) and parasagittal (B)planes on day 0. MDCT images showed contrast medium in the aortic wall of the aortic root and ascending aorta. Contrast medium is also visible in the pericardial recess (A, arrowhead).
How would I treat?
The Invited Experts’ opinion
This case is a catheter-induced intramural haematoma of major size located in the ascending aorta. It’s evolution is naturally related –as well as caused– by iatrogenic manipulation with instruments in the anatomic vicinity of the right coronary orifice; thus, we are dealing with the rapid evolution of an intramural haematoma with intimal disruption (or defect, even if not identified on CTA) in other words an iatrogenic type A dissection with a class V intimal tear and wide separation of aortic layers.
Considering that the guideline recommendation (Class I, LoE B) for the definitive treatment of acute ascending dissection and intramural haematoma with intimal disruption requires urgent evaluation and surgical repair due to the high risk of associated life-threatening complications (rupture, tamponade), one may not hesitate to send the patient to the operating theatre. However, in a scenario of a pinpoint intimal laceration at the proximal RCA feeding the growing wall haematoma, I would seal that local entry with a coronary stent-graft placed into the proximal RCA, downgrading the emergent problem to an IMH without intimal defect, probably stopping further growth (as done by the authors). With a successful intervention, we are dealing with a class II A (LoE C) recommendation, treating such IMH similar to aortic dissection in a corresponding aortic segment and buying some time for elective surgery. Nevertheless, this case remains a DeBakey II problem, and the entire dissected segment needs an interposition or conduit graft.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
The Invited Experts’ opinion
The right coronary artery (RCA) presents a dissection that propagated up to the ostium and ascending aorta and was identified by a dense staining of the aortic wall with incomplete dye clearance after injection of contrast media.
According to the classification proposed by Dunning et al1, it is a class III dissection (extension in the aorta longer than 40 mm).
It is a rare, but impressive and potentially life-threatening complication, probably related in this case to the engagement of the tip of the Amplatz guiding catheter, in spite of the soft manipulation underlined by the authors.
In the experience of the Clinique Pasteur in Toulouse between 1999-2002, over a total of 7,359 percutaneous coronary intervention (PCI) procedures, we identified four cases of such complications (0.05%), all occurring during PCI for RCA lesions: one Dunning classI (limited to the sinus of Valsalva), one class II (extension in the aorta less than 40 mm), and two class III (extension in the aorta greater than 40 mm) (J. Marco, ACC lecture, 2002).
These four cases were successfully stabilised without sequela by a conventional bare metal stent (BMS) implant covering the ostium of the RCA and followed by a conservative management with transesophageal echography (TEE) control and follow-up.
Based on this experience, and experiences reported in the literature2-8, we would manage this complication by sealing the entry site with a BMS implant covering correctly the ostium of the RCA, with the proximal part of the stent protruding within the aorta, followed by a conservative management guided by TEE control.
The key technical points to underline are:
–withdrawing the tip of the guiding catheter within the aorta
–maintaining the guidewire in place in the coronary lumen
–accurately placing the proximal part of the stent within the aorta so as to avoid a large amount of contrast media injections within the coronary ostium that may aggravate the retrograde extension of the dissection.
We would place a 20×3.5mm BMS with at least 2 mm of the proximal part (two struts) protruding within the aorta before the deployment. This accurate placement of the proximal part of the stent within the aorta is mandatory and requires several controls in multiple views without the engagement of the tip of the guiding catheter within the coronary ostium.
The deployment of the stent should be performed only after ensuring the following condition, that at least 2mm of the stent is protruding within the aorta, so that we are sure that, after the balloon inflation (12-14 atmospheres) the stent correctly covers both the ostium of the RCA and the entry site of the aorta dissection.
Regarding the stent type, in reported experiences, conventional BMS have been placed. Is the use of a covered stent more secure? We did not find any answer in the literature.
After the stent deployment, a control by IVUS may be helpful in order to be sure that the stent correctly covers both the RCA ostium entry site and the distal part of the dissection, and that it is also correctly expanded.
Then, at the end of the procedure, on the table, we would check the dissection with a TEE control; TEO is available in all centres and is easy to perform. This will allow the accurate evaluation of the length, extent and severity of the intramural haematoma in the aortic arch.
This first TEE imaging will be the first reference. Further repeated controls would allow us to check the evolution.
In this reported case, the control has been performed one hour after the procedure by using ECG-gated multidetector computed tomography (MDCT). It is an efficient imaging technique. However, MDCT requires mobilising the patient, potentially giving the patient new injections of contrast medium and repeatedly exposing the patient to radiation for further controls.
Another option may be to use magnetic resonance imaging (MRI) as a control if it is easily available. MRI does not require contrast medium injections and does not expose the patient to radiation.
Then, we would recommend managing the patient conservatively in the Intensive Care Unit (ECG, clopidogel, aspirin, other medication and enzymes controls as for routine PCI).
If the patient remains symptom and ECG free, a new control by TEE (or other imaging modalities as previously discussed) is recommended on day three that we evaluate the stabilisation, the favourable evolution or potential worsening of the intramural haematoma.
In case of any symptom, transthoracic and TEO may be immediately performed. If the intramural haematoma remains stable and the dissection sealed, the patient may be discharged between day 5-7 with a new TEE (or MRI) planned at one month follow-up, or earlier if symptoms occur. A clear, comprehensive patient history is required.
In our experience, and in the majority of reported cases, sealing the entry site with a stent followed by a conservative approach was sufficient to resolve this complication without sequela.
Emergency surgical repair will only be proposed for life-threatening evolutive extension (evaluated by sequential TEE or MRI) of the intramural haematoma and dissection to the horizontal arch and cranial branches uncontrolled, despite rest and medically controlled low blood pressure.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
Actual treatment and management of the case
The patient was admitted to the ICU for monitoring. Clinical tolerance was very good, and conservative management was deemed the best strategy after a discussion among interventionists, cardiologists, and surgeons based on previously described cases.1-3 On day five, MDCT showed a marked decrease in haematoma size (Figures3, A and B), and the patient was discharged.
After four months, the aortic wall was normal by MDCT (Figure3, C and D). Persistent angina prompted a coronary angiogram, which showed recanalisation of the RCA at the occlusion site and patency of the covered stent. Transthoracic echocardiography showed a normal left ventricle ejection fraction and no regional contractile dysfunction. Angioplasty of the chronic total occlusion of the mid-portion of the RCA at the site of the previous occlusion was achieved using two drug-eluting stents (2.5mm; 28mm and 33mm in length, respectively; Cypher Select®, Cordis). The patient was discharged from the hospital on the third day.
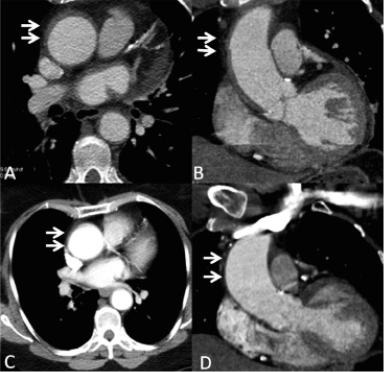
Figure 3. Multidetector computed tomography (MDCT) images of the ascending aorta in the axial (A, C) and parasagittal (B, D) planes. MDCT five days after angioplasty evidenced a residual intramural haematoma in the aortic wall (A and B arrows). Four months later (C and D, arrows), the aortic wall was normal by MDCT.
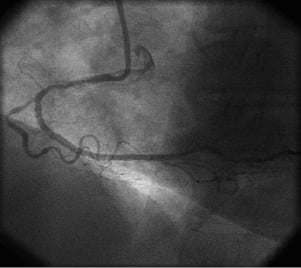
Figure 4. Coronary angiography. A: 40° left oblique view: angiography four months later after successful angioplasty of the previously occluded RCA segment.
To the best of our knowledge, this is the second report3 of conservative management of a large iatrogenic intramural haematoma of the ascending aorta extending to the brachiocephalic artery. Acute dissection of the ascending aorta is a very rare complication of coronary angiography or angioplasty (0.03%), and most cases are limited to the aortic root.4 The recommended treatment for acute dissection of the ascending aorta (in an artery with an abnormal media and diseased vasa vasorum5) is aortic surgery (European Society of Cardiology, ACC/AHA Guidelines),6 but there are no guidelines for cases of complicated coronary artery dissection during PCI. There have been several reports of small iatrogenic haematomas of the aortic root treated successfully with covered stent implantation.4 However, treatment decisions are largely based on experience in each cardiology centre. In our patient, the haematoma was due to a tear in the aortic wall case caused by the guiding catheter. Covered stent implantation was successful, despite the large size of the haematoma.
Our case emphasises the importance of clinical monitoring and cardiac MDCT. Watchful waiting allowed us to avoid cardiac surgery. Conversely to a recent report3 of successful conservative management in a patient with iatrogenic dissection of the ascending aorta, we performed PCI only after the healing of the aortic dissection. Both strategies may be reasonable, except in patients with symptomatic myocardial ischaemia.
In conclusion, as the pathophysiology of iatrogenic dissection complicating PCI seems different from that of atheromatous dissection, a reasonable strategy may be the conservative management by closing the entry site injury with a coronary stent, followed by close clinical and MDCT monitoring in the ICU. This strategy requires close collaboration with a surgical team.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. RCA and aortic dissection.
Moving image 2. Angiography after covered stent.
Moving image 3. RCA angioplasty four months later.

