Abstract
Aims: Percutaneous coronary intervention (PCI)-related risks are increased among patients with left main disease (LMD). The aim was to evaluate the impact of antithrombotic therapy on outcomes after LMD PCI in a predominantly ACS population.
Methods and results: One hundred and seventy-seven patients undergoing LMD PCI were identified in a pooled dataset of 14,326 patients from three large randomised trials comparing treatment with heparin plus glycoprotein IIb/IIIa inhibitors (GPI) or bivalirudin alone, including the REPLACE-2, ACUITY and HORIZONS-AMI trials. Overall, net adverse clinical events (NACE) and non-CABG major bleedings at 30 days occurred more frequently in patients undergoing LMD PCI compared to the overall non-LMD PCI population (NACE: 19.8% vs. 10.6%, p≤0.001, major bleeding: 9.6% vs. 4.6%, p≤0.001). In the LMD group, bivalirudin was associated with significantly less non-CABG related major bleeding compared to heparin+GPI (4.5% versus 14.6%, relative risk [RR] 0.27, 95% CI: 0.09-0.83; p=0.013), while the composite ischaemic endpoint (death/MI/TVR) at 30 days was similar in the two groups (11.4% vs. 12.4%, p=0.513) resulting in a benefit on NACE for bivalirudin over heparin+GPI (14.8% vs. 24.7%; RR 0.53; p=0.039).
Conclusions: Among patients undergoing LMD PCI, the use of bivalirudin instead of heparin+GPI resulted in significantly less major bleeding and improved short-term net clinical outcome. Bivalirudin may be the preferred anticoagulation strategy in LMD PCI patients.
Abbreviations
BMS: bare metal stent
CABG: coronary artery bypass graft
CAD: coronary artery disease
DES: drug-eluting stent
GPI: glycoprotein IIb/IIIa inhibitors
LMD: left main disease
PCI: percutaneous coronary intervention
PTCA: percutaneous transluminal coronary angioplasty
Introduction
Traditionally, left main disease (LMD) represented an absolute indication for coronary artery bypass surgery (CABG). However, for patients with cardiogenic shock or reduced life expectancy due to concomitant severe diseases, interventional treatment for LMD became an alternative option1,2. Advances in device technology, and especially the increasing adoption of drug-eluting stents (DES), have expanded interventional treatment of LMD, even among patients eligible for CABG3-5. Although the optimal treatment for LMD is still a widely debated and controversial topic, encouraging intermediate-term results with DES implantation reported in registries and confirmed by small randomised trials have led to a more widespread performance of percutaneous coronary interventions (PCI) for LMD. The choice of antithrombotic therapies in patients undergoing PCI may have a significant impact on clinical outcomes, especially among high-risk ACS patients who have markedly elevated levels of thrombin and increased thrombin activity. However, anticoagulation may also contribute to bleeding complications with an established impact on morbidity and mortality6. To date, there is insufficient evidence about the optimal antithrombotic strategy during PCI for LMD that would optimise the delicate balance of ischaemic protection without an increase in bleeding. The present analysis aims to evaluate the impact of antithrombotic treatment strategy on short-term clinical outcomes after LMD PCI from a pooled data set from three large randomised trials.
Methods
STUDY POPULATION
The study population was derived from a pooled data set of 14,326 patients treated with elective, urgent or emergent PCI in three large randomised trials, and the current analysis centres on a subgroup of 177 patients undergoing index PCI for LMD. The three trials were the Randomized Evaluation in PCI Linking Angiomax to reduced Clinical Events 2 (REPLACE-2) trial, the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial, and the Harmonizing Outcomes with RevascularIZatiON and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. The contribution to the LMD cohort from each trial is shown in Figure 1. Trial designs and the principal results have been published elsewhere7-9. Briefly, in the REPLACE-2 trial, 6,002 patients with stable angina, inducible ischaemia, or unstable coronary syndromes were blindly randomised to receive either intravenous (IV) bivalirudin (0.75 mg/kg bolus plus 1.75 mg/kg/hour for the duration of PCI) with provisional glycoprotein IIb/IIIa inhibitors (GPI) use or unfractionated heparin (UFH) (65 U/kg bolus) plus GPI (abciximab or eptifibatide). All patients were treated with aspirin. Pre-treatment with clopidogrel was strongly encouraged and was administered two to 12 hours before PCI, followed by a daily maintenance dose of 75 mg for at least 30 days. ACUITY was a randomised open-label, active-comparator trial, including 13,819 patients with moderate to high-risk ACS who were managed by an early invasive strategy, allocating them to one of the three treatment arms: heparin (UFH or enoxaparin) plus GPI (abciximab, eptifibatide, or tirofiban), bivalirudin plus GPI, or bivalirudin alone with provisional GPI use as bail-out strategy. Bivalirudin was started prior to angiography with an IV bolus of 0.1 mg/kg and an infusion of 0.25 mg/kg/hour. In patients triaged to PCI, prior to intervention, an additional IV bolus of 0.5 mg/kg was given, and the infusion rate increased to 1.75 mg/kg/hour. Aspirin 300-325 mg PO or 250-500 mg IV was administered daily during the index hospitalisation, followed by indefinite maintenance dosing of 75–325 mg daily. Dosing and timing of clopidogrel were left to the discretion of the investigator, although a loading dose ≥300 mg was strongly recommended no later than two hours after PCI. Clopidogrel 75 mg daily was recommended for one year. In HORIZONS-AMI, 3,602 patients with ST-segment elevation MI who presented within 12 hours after symptom onset in whom primary PCI was intended were randomly assigned by open-label design in a 1:1 ratio to treatment with UFH plus GPI or to bivalirudin alone for the duration of PCI. UFH was given as an IV bolus of 60 IU/kg of body weight and additional boluses to achieve an activated clotting time of 200 to 250 seconds. Bivalirudin was administered as an IV bolus of 0.75 mg/kg, followed by an infusion of 1.75 mg/kg/hour. The use of GPI was restricted in the bivalirudin group only as bail-out strategy in case of no reflow or high thrombus burden. Either abciximab or double-bolus eptifibatide was allowed at the discretion of the investigator with the infusion maintained for 12 hours (abciximab) or 12 to 18 hours (eptifibatide). A loading dose of clopidogrel (either 300 mg or 600 mg, at the discretion of the investigator) was administered before catheterisation followed by 75 mg orally per day for at least six months. Primary endpoints for this analysis included rates of death, MI, TVR, unplanned revascularisation protocol and TIMI non-CABG-related major and minor bleedings at 30 days. Composite primary endpoints were composite ischaemic events (death, myocardial infarction or unplanned revascularisation) and net adverse clinical events (composite ischaemic events or protocol major bleeding). Major bleeding in both ACUITY and HORIZONS-AMI was defined as any of: intracranial bleeding, intraocular bleeding, access-site haemorrhage requiring intervention, haematoma more than 5 cm in diameter, reduction in haemoglobin concentration of more than 4 g/dL without an overt source of bleeding, reduction in haemoglobin concentration of more than 3 g/dL with an overt source of bleeding, reoperation for bleeding, or use of any blood-product transfusion. In REPLACE-2, major bleeding was defined as intracranial, intraocular, or retroperitoneal haemorrhage, clinically overt blood loss resulting in a decrease in haemoglobin by >3 g/dl, red cell transfusion of ≥2 units or any decrease in haemoglobin >4 g/dl without an overt source of bleeding. Minor bleeding was classified as any bleeding not falling under the definition of major bleeding. Secondary bleeding endpoints included TIMI non-CABG-related major and minor bleedings in all trials. A clinical events committee blinded to treatment assignment adjudicated all 30-day events using original source documents.
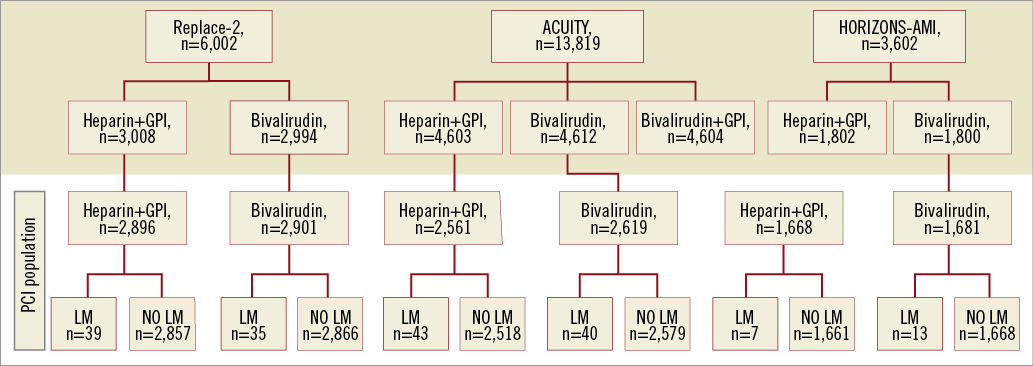
Figure 1. Flow chart showing selection and composition of analysed cohort.
STATISTICAL ANALYSIS
Continuous variables are expressed as means±standard deviation and compared by ANOVA with study in the model, and categorical variables are presented as numbers and percentages of patients and compared with the Cochran-Mantel-Haenszel test adjusting for study. Survival data are presented as Kaplan-Meier curves, and differences between cumulative survival data among treatment groups were tested with the log-rank test. Heterogeneity among the relative risks (RRs) was examined using the Breslow-Day test. For all comparisons, a two-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Among a total of 14,326 patients undergoing PCI in the three trials, left main PCI was performed in 177 patients (1.2%), including 74 of 5,797 (1.3%) patients in the REPLACE-2 trial, 83 of 5,180 patients (1.6%) in the ACUITY trial, and 20 of 3,349 patients (0.6%) in the HORIZONS-AMI trial. Patients with LMD had a higher-risk baseline profile compared to those without LMD, including being older and suffering from more extensive coronary artery disease (Table 1). Baseline clinical and angiographic characteristics as well as procedural details of patients with LMD PCI stratified according to randomised antithrombotic treatment are shown in Table 2. There were no major differences between randomised treatment groups (heparin+GPI vs. bivalirudin) in the baseline demographics among LMD patients. As expected, the incidence of post-PCI ischaemic events, protocol major non-CABG-related bleeding and one-year mortality was significantly higher in patients with LMD compared to patients without LMD (Table 3). Table 4 summarises 30-day post-PCI clinical outcomes of patients with LMD according to randomised antithrombotic treatment. Eight of 88 (9%) patients randomised to bivalirudin monotherapy received provisional GPI (eptifibatide in four patients, abciximab in three patients and tirofiban in one patient). Heterogeneity among the RRs was tested using the Breslow-Day test and the three studies included in this pooled analysis were found to be homogenous for all cases (Breslow-Day value >0.05).
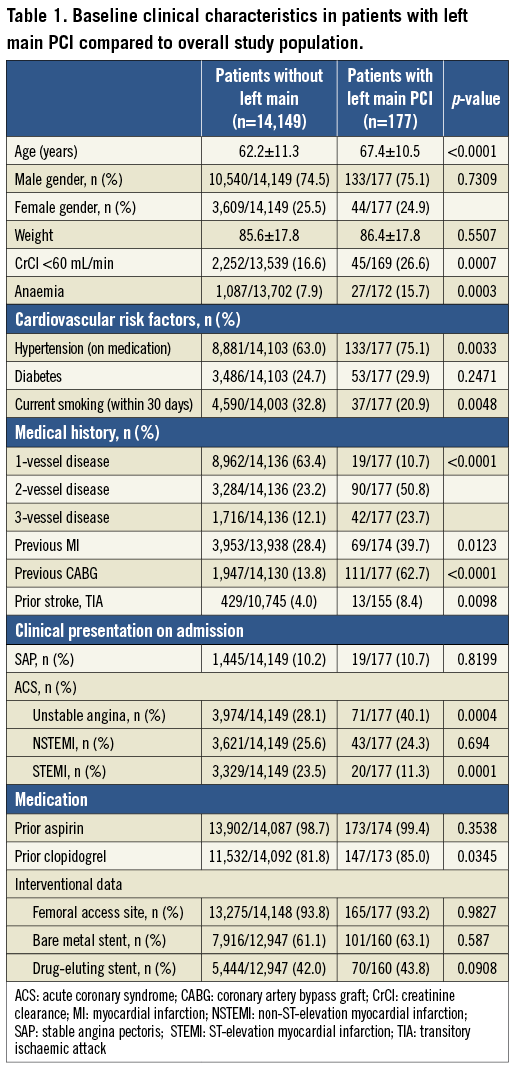
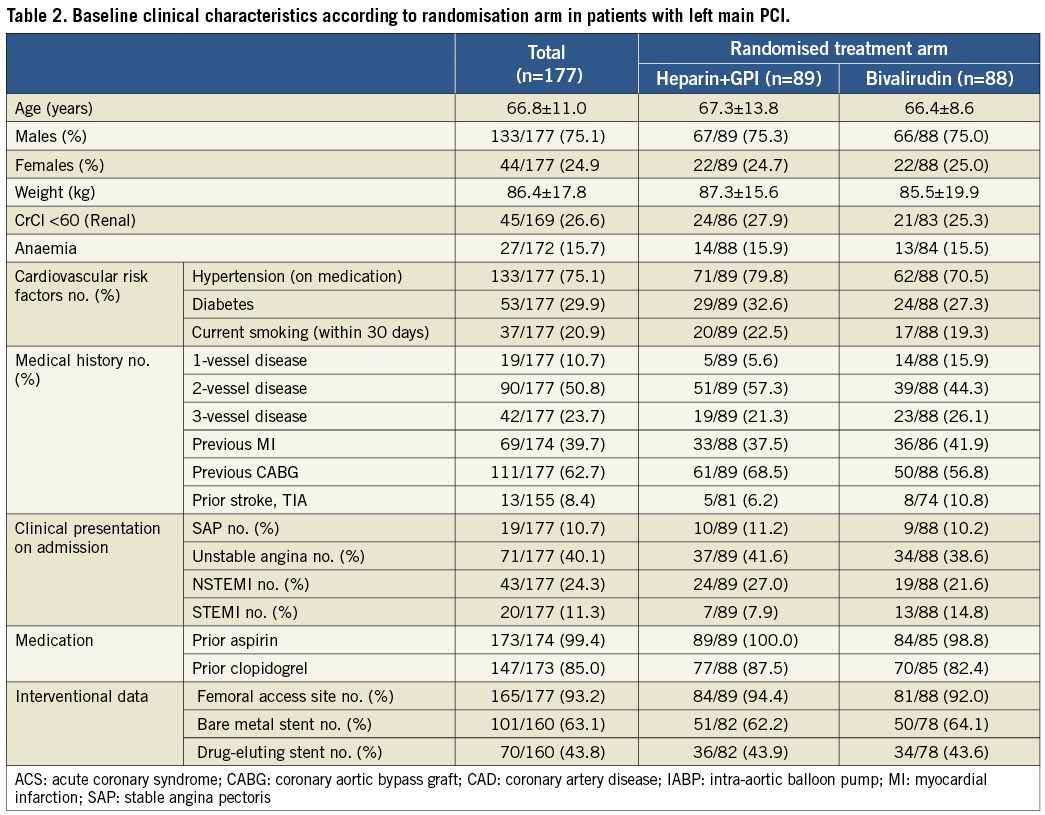
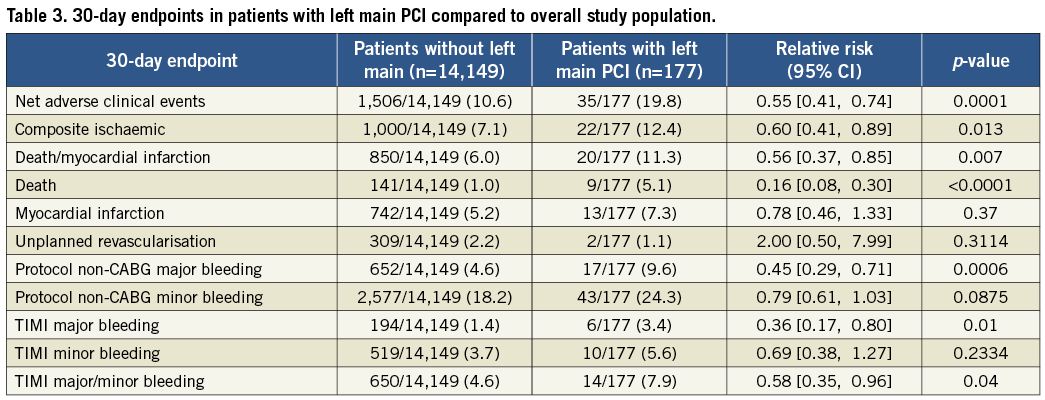
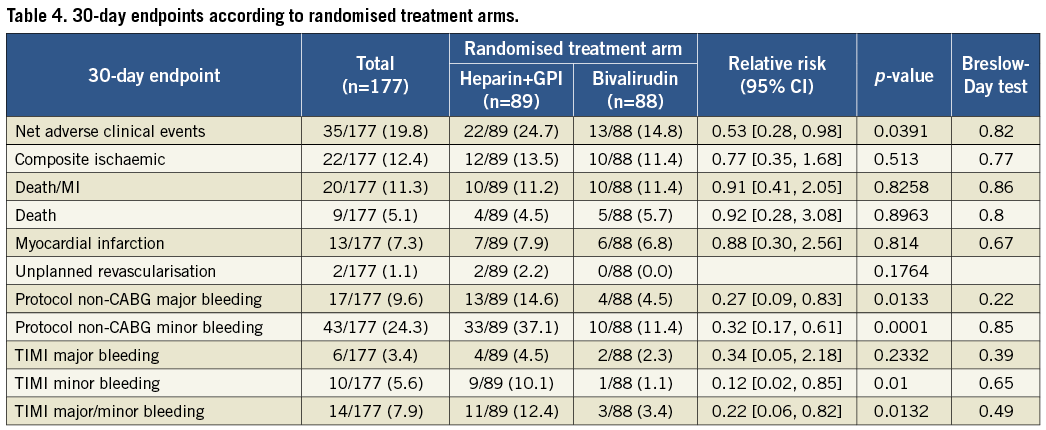
Patients treated with heparin+GPI had significantly higher rates of 30-day net adverse clinical events (NACE: death, myocardial infarction, unplanned revascularisation and protocol non-CABG major bleeding). Kaplan-Meier curves showed similar cumulative event rates for combined ischaemic events (death, myocardial infarction or unplanned revascularisation) (Figure 2) and a significant reduction of protocol non-CABG-related major bleeding in the bivalirudin compared to the heparin plus GPI treatment arm at 30 days (Figure 3). Effects of treatment arms on NACE for the individual trials are shown as a forest plot in Figure 4 (Breslow-Day heterogeneity p=0.82). The incidence of stent thrombosis (ST) in the overall pooled patient cohort was low. Data on ST were not collected in the REPLACE-2 trial (n=74). From the 103 patients in ACUITY and HORIZONS-AMI, only two cases of probable stent thrombosis occurred in the heparin arm of ACUITY. Protocol non-CABG-related major bleeding, minor bleeding and TIMI major/minor bleeding were all significantly lower in bivalirudin-treated patients (Table 4).
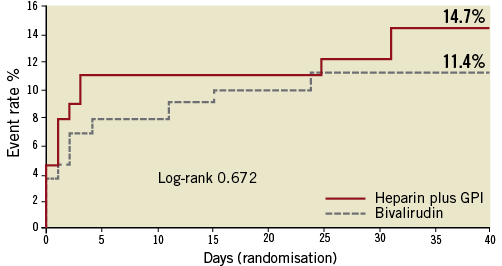
Figure 2. Kaplan-Meier curves for combined ischaemic events (mortality, MI or unplanned revascularisation) at 30 days in ITT population with left main disease according to treatment arms.
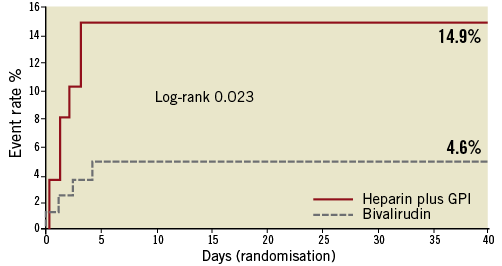
Figure 3. Kaplan-Meier curves for protocol non-CABG-related major bleeding at 30 days in ITT population with left main disease according to treatment arms.
Figure 4. Forest plot showing ORs for the effect of treatment on NACE in individual randomised trials.
Discussion
The principal finding of this subgroup analysis from three randomised trials is that patients who undergo PCI for LMD and receive bivalirudin have rates of ischaemic complications comparable with patients who receive UFH+GPI, but experience significantly fewer bleeding complications. Specifically in this LMD cohort, the choice of bivalirudin instead of UFH+GPI was associated with a 68% reduction in protocol non-CABG major bleeding and a 78% reduction in TIMI major and minor bleeding. Importantly, the results of this subgroup analysis are consistent with the overall results in the three individual trials that demonstrated robust reductions in bleeding complications and equivalent ischaemic protection with the use of bivalirudin compared with UFH+GPI.
As expected, our cohort of patients undergoing PCI for LMD were a higher risk group and experienced higher 30-day event rates than patients without LMD, including a fivefold increase in 30-day mortality (1.0% vs. 5.1%, p<0.0001). Major bleeding complications were especially frequent in the LMD cohort and more than double that in the overall population (4.6% vs. 9.6%, p=0.0006). The high risk for both ischaemic and bleeding events in patients with LMD is most likely related to their advanced age and very frequent comorbidities. In our cohort of 177 patients, 30-day outcomes were comparable to those reported by registries reporting on LMD PCI10. Recent studies suggest that overall mortality rates after LMD PCI are similar to CABG; however, when applying the SYNTAX score for risk stratification, it appears that patients with higher scores (>33) have a survival advantage with CABG11-13. Thus, LMD PCI should be viewed as a reasonable and safe treatment option in low to moderate-risk patients and efforts should focus on improving outcomes further in this group.
The optimal antithrombotic strategy for LMD PCI has not been characterised and may represent an important opportunity for improving outcomes in these high-risk patients, and specifically in relation to the very high rates of bleeding14. A major bleed after PCI has been convincingly linked to both short and long-term mortality, but also to an increased risk for stent thrombosis15-17. In addition, periprocedural bleeding may lead to haemodynamic compromise caused by hypovolaemia, anaemia, hypotension and reduced oxygen-carrying capacity. In patients with LMD, such complications are most undesirable as they could rapidly lead to a fatal outcome, and recent reports demonstrated that stable haemodynamics after LMD PCI are associated with improved outcome18,19. In addition, transfusions that are frequently used to manage bleeding complications have their own independent and substantial impact on mortality20-23. Finally, there appears to be a correlation between incidence of major bleeding and level of residual SYNTAX score as demonstrated in a recent report24. It is tempting to speculate that the higher mortality rates in patients with high SYNTAX scores may in part be explained by the higher bleeding rates and that in such patients an antithrombotic strategy that reduces bleeding risk may be particularly beneficial. The ability to reduce major bleeding rates can therefore have substantial clinical benefits and could contribute to substantial cost savings. The biologic explanation for a differential treatment of effect according to lesion location is speculative. However, LMD is a powerful surrogate for a host of other clinical characteristics that may be associated with increased risk for post-procedural events, and could explain an even greater magnitude of benefit for bivalirudin, including older age, more frequent low EF or CHF and more extensive CAD. Thus, it has been previously reported that bivalirudin benefits increase with increasing periprocedural risk25.
Limitations
The present analysis has several limitations. First, it is a post hoc subgroup analysis of three major randomised trials with all its shortcomings and pitfalls. The sample size of this analysis of 177 patients with LMD PCI is modest and not sufficiently powered to evaluate all clinical endpoints including mortality. However, we did observe a highly significant difference in bleeding rates using either the protocol definition or the TIMI classification. Additionally, this study was not adjusted for heterogeneity due to different study design and inclusion criteria of the three randomised trials. However, there was no significant difference regarding clinical, demographic and interventional factors between both treatment groups in the subgroup of patients undergoing left main PCI (Table 1), and most importantly the outcome results reported from this subgroup are entirely consistent with those reported in each of the three adequately powered trials. The additional risk associated with the complexity of coronary artery disease cannot be fully provided, therefore an estimation of risk benefit of the antithrombotic treatments according to baseline SYNTAX score cannot be assessed in this cohort.
Conclusions
In conclusion, the present analysis from a pooled cohort of patients undergoing PCI for LMD suggests that procedural anticoagulation with bivalirudin compared to UFH+GPI significantly reduces bleeding complications and preserves protection against ischaemic complications. The benefit observed with the use of bivalirudin in LMD PCI is at least of the magnitude of the benefit of the total population if not greater. Prospective evaluation of the impact of antithrombotic therapies on the high bleeding risk inherent in LM PCI and the impact on post-PCI outcomes of these jeopardised patients is warranted.
Conflict of interest statement
T. Geisler and M. Gawaz have received lecture honoraria from Eli Lilly/Daiichi Sankyo, The Medicines Company, Bayer Health Care and a restricted grant from Eli Lilly/Daiichi Sankyo. G. Dangas and R. Mehran are on the advisory board of and consult for Abbott, AstraZeneca. M. Lincoff received research grants from Eli Lilly, Takeda, Roche, Merck, Regado and Pfizer. E. Deliargyris and D. Bernstein are employees of The Medicines Company. G.W. Stone has served as a consultant to Boston Scientific, Eli Lilly, and Daiichi Sankyo. The other authors have no conflicts of interest to declare.

