
Abstract
Aims: The aim of this multicentre study was to investigate the in-hospital and midterm outcomes of rotational atherectomy (RA) followed by metallic stent implantation.
Methods and results: Between 2002 and 2013, 1,176 de novo lesions with calcified coronary lesions treated by RA and metallic stent implantation at nine institutions were assessed. Patients with ST-segment elevation myocardial infarction (STEMI) within 30 days, cardiogenic shock before the procedure, lesions with thrombus, and in-stent restenosis were excluded from the current analysis. In-hospital major adverse cardiac events (MACE) occurred in 8.3% of cases, mainly driven by periprocedural myocardial infarction. The incidence of MACE was 16.0% at one-year and 24.9% at two-year follow-up, both driven by target vessel revascularisation (13.5% at one year and 19.8% at two years). Multivariable analysis revealed that dialysis was an independent predictor for both in-hospital MACE (OR 2.33, 95% CI: 1.11-4.87, p=0.03) and follow-up MACE (HR 4.14, 95% CI: 2.87-5.96, p<0.001), whilst drug-eluting stent (DES) use was associated with a reduction in follow-up MACE (HR 0.42, 95% CI: 0.26-0.67, p<0.001).
Conclusions: RA appears to be safe and effective with acceptable in-hospital and follow-up MACE considering the severity of patient and lesion characteristics. DES implantation following RA was associated with a reduction in MACE during the follow-up period.
Introduction
Percutaneous coronary intervention (PCI) for severely calcified lesions remains a challenge and, in spite of procedural and technical advances, continues to be associated with lower procedural success and higher complication rates when compared to non-calcified or mildly calcified lesions1,2. The main reasons for this include device delivery failure, incomplete stent expansion, malapposition of stent struts, and resultant higher rates of restenosis3-5. Percutaneous rotational atherectomy (RA) emerged in the late 1980s6,7 and was initially considered as an adjunctive tool to reduce plaque volume in the era of plain old balloon angioplasty6,8. However, following the advent of coronary stents, some randomised trials and retrospective studies did not show an additional benefit of RA over conventional PCI with stenting alone with regard to long-term outcomes4,9,10.
In contemporary PCI practice, RA is considered as an adjunctive tool for the management of fibrotic or heavily calcified coronary lesions in instances when a balloon catheter does not pass or the lesion cannot be adequately dilated before stent implantation11-13. RA is currently used in 1-3% of all PCI procedures13. In the ROTAXUS (Rotational Atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease) study, which randomised moderate to severe calcified lesions to either routine or provisional RA9, the crossover rate to RA treatment in the provisional RA group was 12.5%. However, there are limited data investigating clinical outcomes following RA use in contemporary clinical practice. We therefore conducted this multicentre, retrospective study to investigate the in-hospital and midterm outcomes of RA followed by metallic stent implantation.
Methods
STUDY POPULATION
Between April 2002 and August 2013, 1,076 consecutive patients with calcified coronary lesions treated by RA at nine different institutions were enrolled into the ROTATE registry. Exclusion criteria included ST-segment elevation myocardial infarction (STEMI) within 30 days, cardiogenic shock before the procedure, and lesions with angiographic evidence of thrombus at the site of the target lesion. Of the total of 1,284 lesions in the 1,076 patients enrolled, 32 lesions with in-stent restenosis, 67 lesions treated without stent implantation and nine lesions treated with bioresorbable scaffolds were excluded from the current analysis (Figure 1). The decision to perform RA was at the operators’ discretion at each centre. Clinical follow-up was performed through hospital records or telephone consultations.
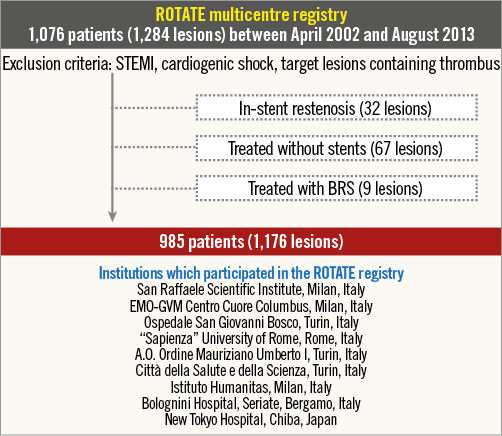
Figure 1. Study design. Of the total of 1,284 lesions (1,076 patients) included in this registry, 1,176 lesions (985 patients) were assessed for eligibility after excluding lesions with in-stent restenosis (32 lesions), those treated without stenting (67 lesions), and those treated with bioresorbable scaffold (BRS; nine lesions). STEMI: ST-segment elevation myocardial infarction
PROCEDURE
All RA procedures were performed using the Rotablator™ rotational atherectomy system (Boston Scientific, Marlborough, MA, USA). Lifelong low-dose aspirin was recommended for all patients in addition to a thienopyridine (75 mg of clopidogrel daily or 200-250 mg of ticlopidine bid) for a minimum of 12 months after DES implantation, and for a minimum of six months after bare metal stent (BMS) implantation. All clinical decisions, such as vascular access, burr size, glycoprotein IIb/IIIa inhibitors or bivalirudin, were at the operators’ discretion.
STUDY DEFINITIONS
In-hospital major adverse cardiac events (MACE) were defined as the combination of all-cause death, any myocardial infarction (MI) (including periprocedural MI), and target lesion revascularisation (TLR) during hospitalisation. At each institute, we defined periprocedural MI as an elevation of creatine kinase-myocardial band more than three times the upper reference limit (URL)14. Follow-up MACE were defined as the composite endpoint of all-cause death, follow-up MI (excluding periprocedural MI), and TLR after the procedure. Follow-up MI was defined as an elevation of cardiac biomarkers (cardiac troponin being the preferred biomarker) with at least one value above the URL, together with evidence of myocardial ischaemia with at least one of the following: ischaemic symptoms, electrocardiogram (ECG) changes indicative of new ischaemia, development of pathological Q-waves on the ECG, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality14. Asymptomatic stroke detected by magnetic resonance imaging or other imaging modalities was not classified as a stroke event during follow-up.
STUDY ENDPOINTS
The primary endpoint was the occurrence of midterm (two-year) MACE. The secondary endpoints were the occurrence of in-hospital adverse events, and each component of follow-up MACE, target vessel revascularisation (TVR), stent thrombosis (ST), and stroke.
QUANTITATIVE CORONARY ANGIOGRAPHIC ANALYSIS
Quantitative coronary angiography (QCA) was performed using standard methods and definitions15 at each institution. Reference vessel diameter, minimum lumen diameter, percent diameter stenosis, and lesion length were measured before and after PCI.
STATISTICAL ANALYSIS
All continuous variables were evaluated with the Kolmogorov-Smirnov test for distribution. Continuous variables are presented as mean±standard deviation for normally distributed variables or median±interquartile range for non-Gaussian distributed variables. Categorical variables are presented as numeric values and percentages. Clinical event rates were calculated using Kaplan-Meier analysis, and the differences between groups were assessed with the log-rank test. All clinical events were analysed on a per-patient basis. Multivariable logistic regression analysis was performed to identify the independent risk factors for in-hospital MACE, and Cox regression analysis for follow-up MACE. Variables entered into the multivariable model were those which reached significance (p<0.10) following univariate analysis and judged to be clinically significant. All reported p-values are two-sided and p-values <0.05 were regarded as statistically significant. Statistical analyses were performed with SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
STUDY POPULATION AND BASELINE CHARACTERISTICS
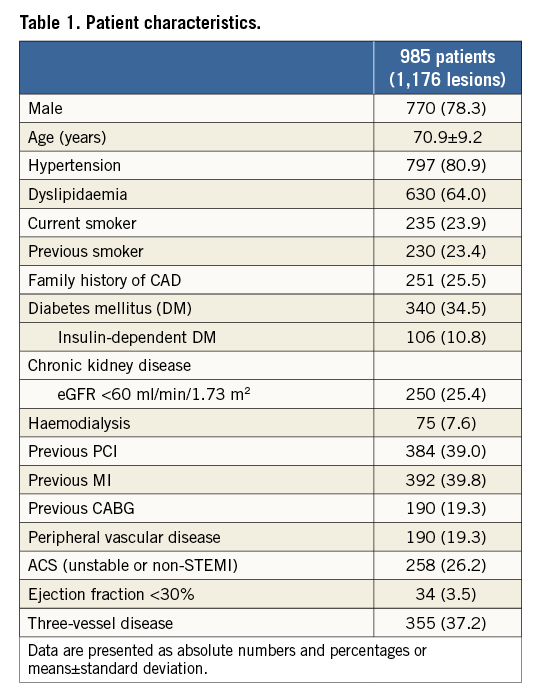
During the study period, 1,176 de novo lesions in 985 patients were assessed for eligibility. The median follow-up period was 573 (interquartile range 320-1,088) days. Baseline clinical characteristics are summarised in Table 1. The prevalence of diabetes mellitus (DM) was 34.5%, and that of acute coronary syndrome (ACS; non STEMI or unstable angina) was 26.2%. Low EF and three-vessel disease were present in 3.5% and 37.2% of patients, respectively. Of note, patients treated with dialysis for end-stage renal failure constituted 7.6% of the total study population.
LESION AND PROCEDURAL CHARACTERISTICS
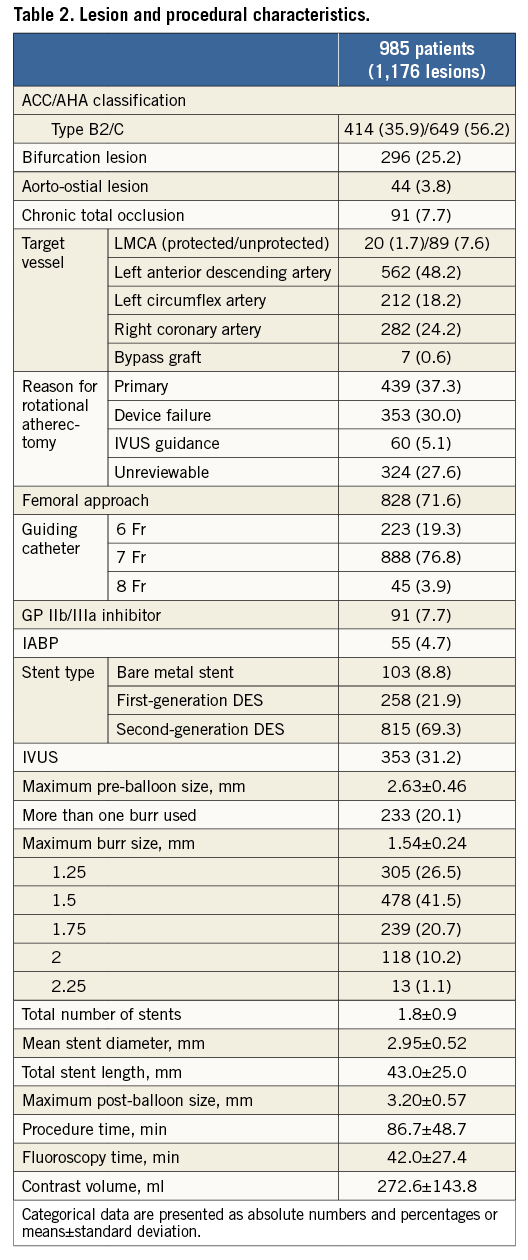
Lesion and procedural characteristics are shown in Table 2. Complex lesion characteristics such as bifurcation, aorto-ostial, or chronic total occlusion (CTO) lesions were present in 25.2%, 3.8%, and 7.7% of patients, respectively. The main target vessel treated with RA was the left anterior descending artery (LAD; 48.2%). Although there was no specific indication for the use of RA in 27.6% of patients, a primary strategy of RA accounted for use in 37.3% of patients, with secondary RA following any device failure such as underexpansion of balloon or uncrossable devices in 30.0%. The transfemoral approach was the most commonly used (71.6%), and the most frequent sheath size was 7 Fr (76.8%). An intra-aortic balloon pump (IABP) was utilised in 4.7% of patients. Of note, 69.3% of lesions were treated with second-generation DES. Stent optimisation using intravascular ultrasound (IVUS) was performed in 31.2% of patients.
PROCEDURAL AND IN-HOSPITAL OUTCOMES
QCA data, procedural and in-hospital outcomes are summarised in Table 3. QCA data were available for 908 lesions (77.2%). Mean reference vessel diameter was 2.78±0.63 mm, and acute gain was 2.29±0.64 mm. Optimal stent expansion (final percent diameter <20%) was obtained in 90.6% of patients.
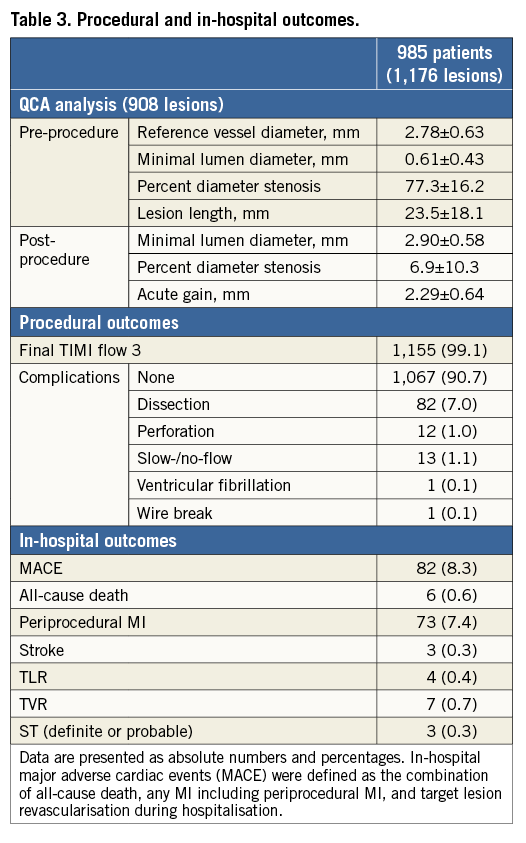
Final TIMI 3 flow was achieved in 99.1% of patients even though slow- or no-flow was observed in 1.1%. More than 90% of cases were treated without any complication. The most common complication during PCI with RA was residual dissection (7.0%). Coronary perforation was observed in 1.0% of all cases, and one patient (8.3%) died as a direct consequence of coronary perforation during hospital stay. In-hospital MI (including periprocedural MI) was detected in 7.4% of patients. In-hospital MACE occurred in 8.3% of patients, which was mainly driven by periprocedural MI. In-hospital death or symptomatic stroke occurred in 0.6% and 0.3% of patients, respectively. The cumulative incidence of acute and subacute ST was 0.3% (n=3) and 1.3% (n=11), respectively.
MIDTERM CLINICAL OUTCOMES
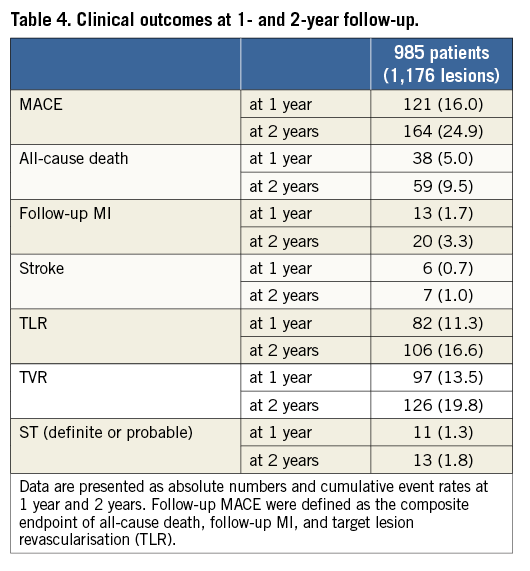
Clinical outcomes at one and two years are summarised in Table 4. The incidence of MACE was 16.0% at one-year and 24.9% at two-year follow-up and was mainly driven by a high rate of TVR (13.5% at one year and 19.8% at two years). All-cause death occurred in 5.0% of patients at one year and 9.5% at two years. Although the majority of cases were treated with drug-eluting stents, the rates of TLR were high, i.e., 11.3% and 16.6% at one- and two-year follow-up, respectively. The cumulative rates of ST were 1.3% (n=11) at one-year, and 1.8% (n=13) at two-year follow-up.
PREDICTORS OF IN-HOSPITAL MACE
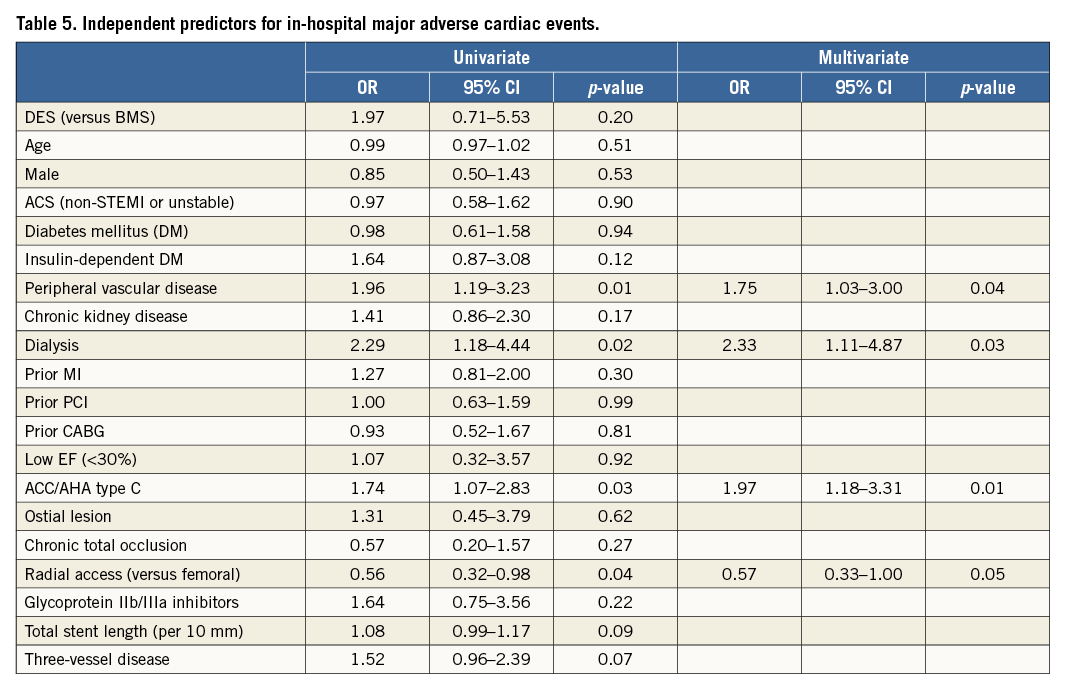
Univariate analysis identified the following risk factors for in-hospital MACE: peripheral vascular disease, dialysis, ACC/AHA type C lesion, and radial access. Multivariable analysis revealed that all significant risk factors in the univariate model remained independent predictors of in-hospital MACE (peripheral vascular disease [OR 1.75, 95% CI: 1.03-3.00, p=0.04], dialysis [OR 2.33, 95% CI: 1.11-4.87, p=0.03], ACC/AHA type C lesion [OR 1.97, 95% CI: 1.18-3.31, p=0.01], and radial access [OR 0.57, 95% CI: 0.33-1.00, p=0.05]) (Table 5).
PREDICTORS OF FOLLOW-UP MACE
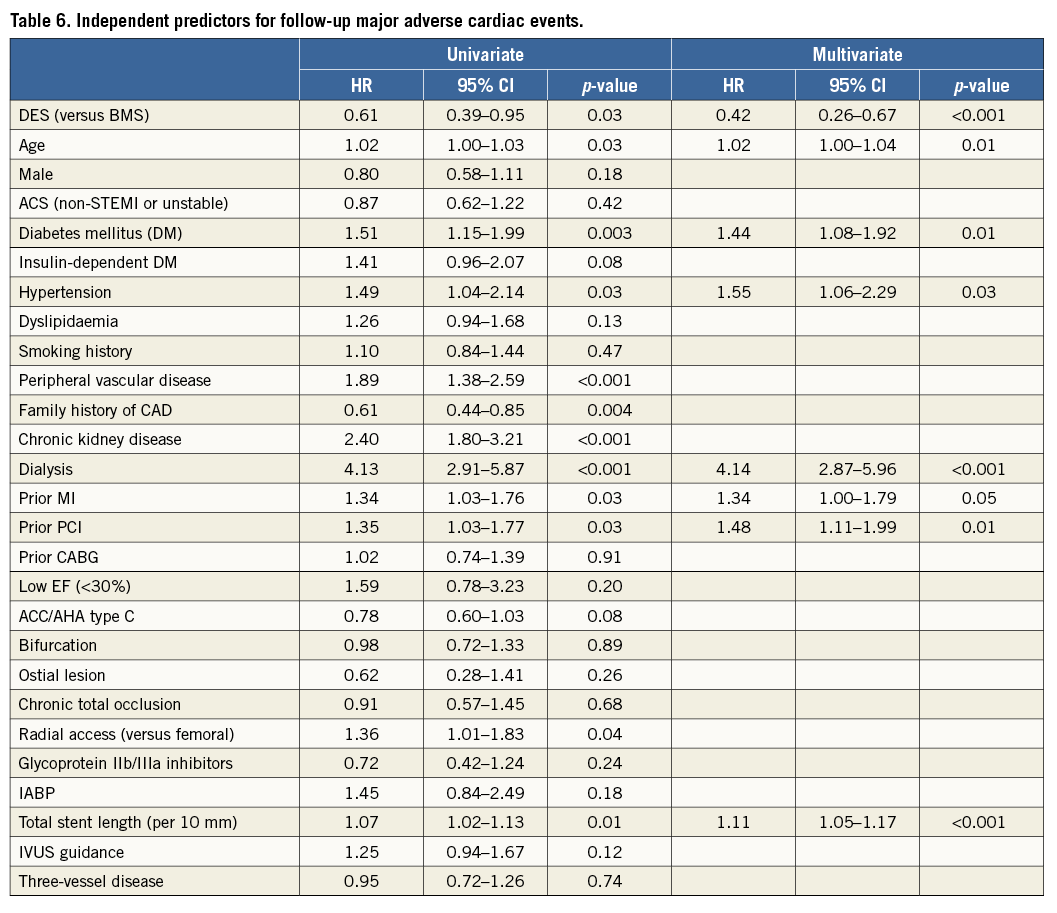
Univariate analysis identified the following risk factors for follow-up MACE: DES (versus BMS), age, DM, hypertension, peripheral vascular disease, family history of coronary artery disease (CAD), chronic kidney disease (CKD), dialysis, prior MI, prior PCI, radial access, and total stent length per 10 mm. Multivariable analysis revealed DES (versus BMS) (HR 0.42, 95% CI: 0.26-0.67, p<0.001), age (HR 1.02, 95% CI: 1.00-1.04, p=0.01), DM (HR 1.44, 95% CI: 1.08-1.92, p=0.01), hypertension (HR 1.55, 95% CI: 1.06-2.29, p=0.03), dialysis (HR 4.14, 95% CI: 2.87-5.96, p<0.001), prior MI (HR 1.34, 95% CI: 1.00-1.79, p=0.05), prior PCI (HR 1.48, 95% CI: 1.11-1.99, p=0.01), and total stent length per 10 mm (HR 1.11, 95% CI: 1.05-1.17, p<0.001) as independent predictors of follow-up MACE (Table 6).
Discussion
This multicentre registry investigated the in-hospital and midterm outcomes of RA followed by metallic stent implantation in the context of contemporary PCI practice. The main findings of this study are the following: 1) in-hospital MACE was observed in 8.3% of cases, which was predominantly driven by periprocedural MI; 2) rates of follow-up MACE were acceptable considering the severity of patient and lesion characteristics; 3) dialysis was identified as an independent predictor for both in-hospital and follow-up MACE; 4) DES use was associated with a reduction in follow-up MACE.
RA has shown excellent results in improving procedural success for the treatment of severely calcified coronary lesions16,17. However, even with the benefit of technological advances such as the introduction of newer-generation DES, this has not translated into a long-term benefit due to the high rates of late loss and restenosis in spite of the larger acute gain achieved following RA use3,9. However, the currently available data may not be applicable to contemporary practice (greater operator experience, advances in stent and balloon technology) because most previous RA studies were conducted in the balloon angioplasty or BMS era.
This multicentre, retrospective study demonstrated acceptable in-hospital outcomes (in-hospital MACE: 8.3%) specifically when considering only patients receiving newer-generation DES following RA. In support of previous studies11, the rate of in-hospital MACE was principally driven by periprocedural MI, although the mortality rate was low (all-cause death: 0.6%). However, the presence of periprocedural MI did not affect one-year MACE (periprocedural MI 18.5% vs. no periprocedural MI 15.8%, p=0.50) and mortality (periprocedural MI 5.0% vs. no periprocedural MI 5.0%, p=0.97). Our study further demonstrated acceptable midterm clinical outcomes following RA regardless of the generation of subsequent metallic stent implanted.
The follow-up MACE that we observed in this registry (16.0% at one year, and 24.9% at two years) was acceptable when bearing in mind the high complexity of lesion and patient characteristics. This was mainly driven by TVR (13.5% at one year, and 19.8% at two years). The rate of all-cause death was 5.0% at one year and 9.5% at two years. Stent underexpansion is considered a risk factor for stent thrombosis18, which is more common when treating calcified lesions. The incidence of stent thrombosis was 1.3% and 1.8% at one and two years, respectively, and was consistent regardless of the type of stent implanted.
Multivariable analysis demonstrated peripheral vascular disease, dialysis and ACC/AHA type C lesions to be independent predictors of in-hospital MACE, whilst the use of radial access was found to be an independent predictor of reduced in-hospital MACE (although we cannot exclude selection bias with regard to the choice of vascular access site). In this study, even though we did not investigate the rate of bleeding complications, there was a significant difference regarding the rate of the composite of in-hospital MACE (femoral 9.7% vs. radial 5.7%, p=0.04), although not for each individual component (in-hospital death; 0.9% vs. 0%, p=0.19, in-hospital MI; 8.5% vs. 5.4%, p=0.11, in-hospital stroke; 0.3% vs. 0.3%, p=1.00, in-hospital stent thrombosis; 0.3% vs. 0%, p=1.00). Cockburn et al reported on the long-term mortality of 2,152 patients treated with RA in the UK between 2007 and 201119. In support of the current data, this study also demonstrated that mortality was linked to the premorbid risk of the patient population rather than the specific procedure itself. These observations demonstrate the importance and impact of patient and lesion characteristics upon in-hospital or follow-up outcomes.
With regard to the impact of the use of DES following RA, multivariate analysis confirmed DES to be associated with a reduction in follow-up MACE. We initially compared clinical outcomes (MACE) in the overall population (Figure 2A) that demonstrated a benefit of DES over BMS; however, further subgroup analysis suggested a possible advantage of the first-generation (1G)-DES over the second-generation (2G)-DES. Potential explanations for this were the mandated follow-up angiography (79.1%) and high prevalence of dialysis patients (26.9%) in one study centre which had a large proportion of patients treated with 2G-DES, which may have resulted in a greater TLR rate. On the basis of this clear difference in the clinical practice between contributing centres, statistical evaluation demonstrated interaction for stent type (BMS, 1G-DES, or 2G-DES, p<0.001) to be significant, whilst there were no differences when assessing dialysis (p=0.28) or routine angiographic follow-up (p=0.30). Considering this significant difference, we compared the MACE rates amongst each stent type (BMS, 1G-DES, and 2G-DES) in centres where routine follow-up invasive angiography was not performed but rather only when clinically indicated (e.g., new onset patient symptoms, evidence of cardiac ischaemia or high index of clinical suspicion for significant coronary disease) – the clinically driven group (Figure 2B), and again found a benefit of DES compared to BMS, but also an advantage of 2G-DES over 1G-DES that would broadly support the published literature20,21.
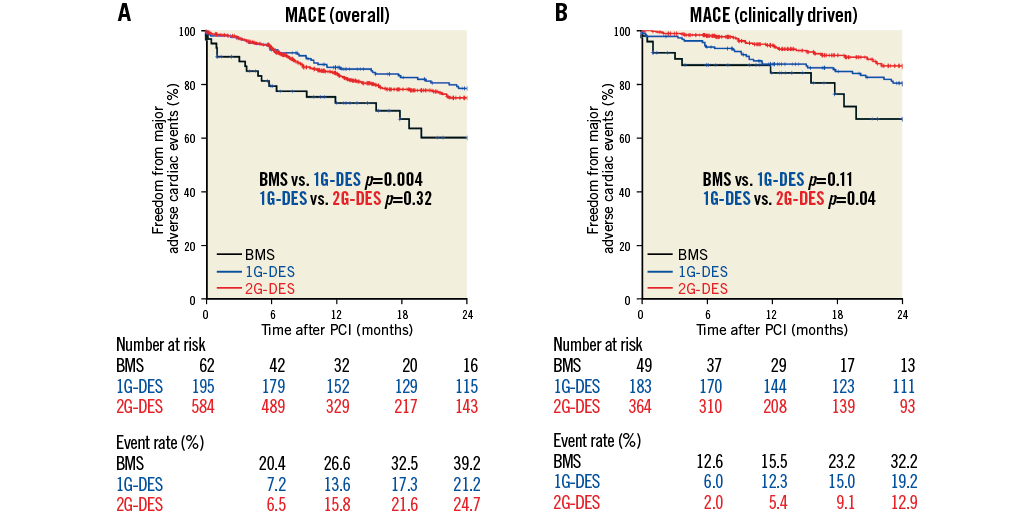
Figure 2. Freedom from major adverse cardiac events between stent types. Kaplan-Meier curves showing freedom from follow-up major adverse cardiac events (MACE) between stent types amongst (A) overall population and (B) clinically driven group. Follow-up MACE were defined as the composite endpoint of all-cause death, follow-up myocardial infarction (MI), and target lesion revascularisation (TLR). BMS: bare metal stents; 1G-DES: first-generation drug-eluting stents; 2G-DES: second-generation drug-eluting stents; PCI: percutaneous coronary intervention
Limitations
As a single-arm retrospective study, this study inevitably carries the limitations inherent to the genre. Several other limitations should be acknowledged. The indication for RA, PCI procedure, or clinical follow-up was dependent on daily practice in each centre, which may have affected the clinical outcomes. Due to the relatively large number of centres included in the study over the long study period, there may be confounders that have not been accounted for. The experience of each operator or centre, temporal technical changes or a learning curve during the study period may have affected outcomes that we could not evaluate in this registry. To ensure that we could include all patients in the analysis, and to account for changes in the definition of MI and tools used to evaluate MI during the long study period (e.g., different biomarkers), we employed different but standardised definitions of MI between periprocedural and follow-up MI. The cause of death was not available for a large proportion of the study population due to the long study period and therefore we only report all-cause mortality without differentiating the subgroup of patients suffering cardiac death. Finally, data regarding lesion complexity (e.g., SYNTAX score, quantitative coronary angiography) were not available, and may have provided greater insights into late clinical events following RA treatment.
Conclusions
In our study RA use in contemporary PCI practice was safe and resulted in acceptable in-hospital MACE (8.3%), which was mainly driven by periprocedural MI and follow-up MACE events, when considering the high patient and lesion complexity. Multivariate analysis identified dialysis to be an independent predictor of both in-hospital and follow-up MACE, with 2G-DES associated with a reduction in MACE during the follow-up period.
| Impact on daily practice Rotational atherectomy (RA) results in high procedural success rates with acceptable short- and longer-term major adverse cardiac event (MACE) rates considering the severity of patient and lesion characteristics. Multivariable analysis identified dialysis to be an independent predictor for both in-hospital and follow-up MACE. Drug-eluting stent (DES) use was associated with a reduction in follow-up MACE. RA use may also be useful to facilitate the treatment of complex coronary lesions with newer technology (e.g., bioresorbable scaffolds). Larger, randomised studies are required to investigate the efficacy of RA further, for both existing and new indications. |
Guest Editor
This paper was guest edited by Emanuele Barbato, MD, PhD, FESC; Cardiovascular Center Aalst, OLV Clinic, Aalst, Belgium.
Conflict of interest statement
A. Latib serves on a Medtronic advisory board. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

