
Rotational atherectomy (RA) was introduced nearly 30 years ago in order to remove coronary atheroma by “debulking” as opposed to conventional plain old balloon angioplasty (POBA) that was meant to enlarge the lumen by displacing the plaque1. The initial enthusiasm around RA was quickly replaced by scepticism following the short-term and midterm results which were far below expectations2.
Percutaneous coronary intervention (PCI) with RA, in fact, was not infrequently characterised by procedural complications (such as large dissections, no-reflow, etc.) and a high restenosis rate (up to 50% in some instances)1. The suboptimal procedural results observed in these early experiences were mostly related to the aggressive debulking RA strategy and to the less efficient antiplatelet therapy regimens used. In addition, many of these RA procedures were stand-alone, followed by POBA or by bare metal stents.
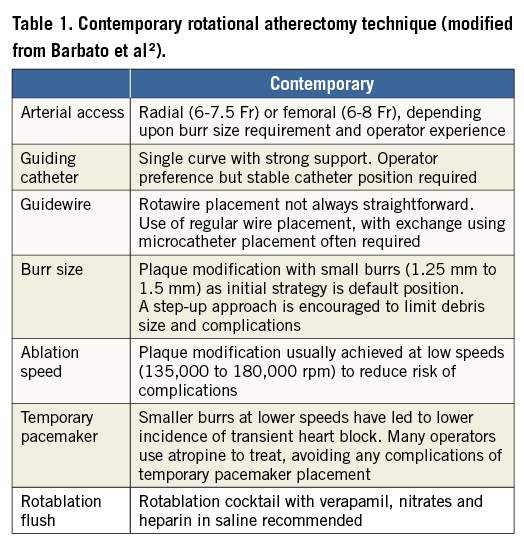
More recently, RA has gone through a second surge of interest, mainly for the following three reasons: 1) PCI is increasingly being performed in heavily calcified coronary stenoses as a consequence of the aging of the patients being referred to the cathlab; 2) excellent results achieved with current-generation drug-eluting stents (DES) have encouraged the performance of PCI in more challenging settings; 3) novel technologies such as bioresorbable vascular scaffolds require more extensive lesion preparation. In addition, the procedural outcome of RA has significantly improved thanks to the evolution from the original extensive “plaque debulking” to the current “plaque modification” technique (Table 1). The contemporary RA technique in fact aims to smoothen the lumen and disconnect the calcified coronary ring, thus leaving the way clear for further balloon dilatation (often with non-compliant or cutting balloons) and stent implantation. This has implied smaller burr-to-artery ratio, lower rotational speed, and burr manipulation, aiming to reduce the friction and temperature increase within the ablated coronary segment (pecking motion technique). In addition to these technical modifications, novel and more potent oral antiplatelet agents have allowed this complex procedure to be performed more safely (less no-reflow, fewer bleedings).
In this issue of EuroIntervention, two reports from the ROTATE registry provide procedural data and clinical endpoints of the RA technique adopted in patients treated from 2002 to 20133,4. As one would expect, patients treated with RA were elderly and at high risk considering that one third had diabetes, one quarter had renal failure (8% treated with dialysis), 37% presented with multivessel disease, and 26% presented with acute coronary syndromes (ACS). The RA technique applied aimed at plaque modification (1.5 mm was the most frequent burr size used), and in 80% of the cases one burr was sufficient. Interestingly, the arterial access used was femoral in 70% of the cases. MACE occurred in-hospital in 8.6% of the patients and was mostly driven by periprocedural myocardial infarction. All patients were treated with classic dual antiplatelet therapy (aspirin plus ticlopidine or clopidogrel). We have no data on the use of bivalirudin in these patients, something which was previously associated with lower periprocedural myocardial infarction in patients undergoing RA5. Whether these results could be improved by the use of more potent P2Y12 inhibitors still remains to be addressed.
Unfortunately, the authors did not provide a temporal trend in the technique applied that would have enabled a better understanding of the impact, for example, of the arterial access used. One might expect an increase in radial access over time facilitated by the adoption of the plaque modification technique that required the use of a single burr, not bigger than 1.5 mm in most of the cases. The association of radial access with lower in-hospital MACE remains an intriguing finding that should be further confirmed. Nevertheless, we cannot rule out that more simple rotablation cases were performed transradially.
Clinical endpoints in these patients were higher than those we are used to seeing for patients undergoing any regular PCI procedure, though they were to be expected considering the coronary anatomy, the clinical condition as well as the complexity of the percutaneous revascularisation (average stent length was 43 mm)6. In particular, in patients presenting with NSTEMI-ACS, the rate of events was not different from that of a matched patient population with NSTEMI treated with PCI without RA4. This suggests that it is rather the intrinsic higher risk of these patients which drives the suboptimal outcome rather than the RA performed. Nevertheless, caution should be used in performing RA in NSTEMI-ACS patients, as suggested by the rate of slow-flow/no-reflow that was twice as high as anticipated, despite the fact that the presence of visible thrombus was an exclusion factor from this registry.
Can we live without rotational atherectomy in these patients? After all, the ROTAXUS trial did not show significant differences in terms of angiographic and clinical endpoints between patients with heavily calcified lesions randomised to conventional PCI versus patients undergoing PCI plus RA7,8. Yet, in the ROTAXUS trial, up to 12% of the patients randomised to conventional PCI crossed over to PCI plus RA. In the ROTATE registry, RA was performed in nearly half of the cases as bail-out strategy, suggesting that, even if avoided in the first place, the procedure could not be performed without the use of the Rotablator™ (Boston Scientific Corp., Marlborough, MA, USA). We can easily anticipate that the outcome in these no-option patients would have been significantly worse if they had not been treated with RA, leading to surgical revascularisation or to higher event rates. The bail-out attitude tends to be replaced by a more judicious elective indication to RA in parallel with operator experience and with increasing confidence with the technique. In fact, relying only on a coronary angiogram might sometimes be limiting, and intracoronary imaging (IVUS was used in 30% of the procedures in the ROTATE registry) can help the operator in deciding when to perform RA electively9. If in doubt, it is better to keep the threshold for RA lower rather than incurring balloon-induced dissections (that would prevent the use of RA) or, even worse, an unexpanded stent. In fact, you will never regret using RA, but you often regret not having used it!
Conflict of interest statement
E. Barbato reports speaker’s fees on his behalf from Boston Scientific to the Cardiovascular Research Center Aalst, Belgium. T. Strisciuglio has no conflicts of interest to declare.

