
Abstract
Aims: The safety and efficacy of rotational atherectomy (RA) in patients presenting with non-ST-elevation myocardial infarction (NSTE-ACS) remain to be defined. The aim of our study was to assess the safety and efficacy of RA in NSTE-ACS patients with reference to both short- and long-term follow-up.
Methods and results: This was an observational retrospective registry which enrolled all consecutive patients undergoing RA, comparing patients with stable angina (SA) and NSTE-ACS. In addition, ACS patients were matched with those not undergoing RA. The primary endpoint was angiographic success. Procedural complications and in-hospital MACE were secondary endpoints along with MACE during follow-up. One thousand three hundred and eight patients were included: 37% (484) with an NSTE-ACS diagnosis and 63% (824) in the SA group. Angiographic success did not differ between the groups (98.8% vs. 99.2%, p=0.57). By univariate analysis procedural complications were more frequent in the NSTE-ACS group (11.3% vs. 8.0%, p=0.04). In-hospital MACE rates were comparable (5.7% vs. 5.8%, p=0.93); by multivariate analysis NSTE-ACS patients showed a non-significant trend towards a higher risk of adverse events (HR 2.39, CI: 0.96-5.96, p=0.061). MACE after a median of 27.9 months was significantly higher in the NSTE-ACS group compared with the SA group (32.4% vs. 24.2%, log-rank p<0.001), results confirmed by multivariate analysis. After propensity score matching, NSTE-ACS patients undergoing RA had similar outcomes to ACS patients who did not undergo RA (16% vs. 13%, log-rank p=0.14).
Conclusions: Rotational atherectomy has similar safety and angiographic outcome in patients with NSTE-ACS or SA. The higher rate of adverse cardiac events at follow-up in NSTE-ACS patients undergoing RA is comparable with a matched population of NSTE-ACS patients not undergoing RA.
Introduction
Acute coronary syndrome (ACS) is the most common reason for cardiac hospitalisation in the western world. Its prevalence is increasing in most eastern European countries and the United States of America. Approximately 70% of these patients experience non-ST-elevation myocardial infarction (NSTE-ACS)1,2.
NSTE-ACS patients are particularly challenging for physicians, as they are older and have more comorbidities than ST-elevation myocardial infarction (STEMI) patients. Furthermore, they frequently present with a more severe pattern of lesions, characterised by multivessel disease with left main involvement2,3, and heavily calcified coronary stenosis that may lead to stent underexpansion and therefore higher rates of stent restenosis or thrombosis4,5.
Cutting balloons and aggressive dilatation6 represent the strategies most used for these lesions, but they may not be enough to avoid an incomplete stent deployment with a consequent increased risk of stent thrombosis7,8.
Rotational atherectomy (RA) may represent a potential solution for these patients. It exerts a “differential cutting” because it acts on non-compliant tissue such as calcified lesions, allowing plaque modification and subsequently more efficient balloon dilatation and stent implantation9.
On the other hand, RA is relatively contraindicated in thrombogenic states such as acute coronary syndromes because of the risk of platelet activation by the rotablator itself10,11. Anecdotal experiences are reported in the literature12,13, but clinical studies are lacking.
The aim of our study was to assess the safety and efficacy of RA in NSTE-ACS patients with reference to both short- and long-term follow-up.
Methods
STUDY POPULATION
This was an observational, retrospective registry. From April 2002 to August 2013, the ROTATE (ROTational AThErectomy) registry was conducted by eight percutaneous coronary intervention (PCI) centres in Italy (“Città della Scienza e della Salute”, Turin, Ospedale San Giovanni Bosco, Turin, San Raffaele Scientific Institute, Milan, Policlinico Umberto I, Rome, Istituto Humanitas, Milan, A.O. Ordine Mauriziano, Turin, Policlinico Umberto I, Rome, Azienda Ospedaliera Bolognini Seriate, Bergamo). Moreover, data of NSTE-ACS patients not undergoing RA from another centre (University Heart Centre, Zurich, Switzerland) were appraised for propensity score matching. Each patient signed informed consent before the procedure and the study was approved by the local ethics committee.
Consecutive patients who underwent rotational atherectomy with the Rotablator™ system (Boston Scientific, Marlborough, MA, USA) to treat one obstructive coronary lesion, defined as more than 70% diameter stenosis at angiography and type B2 or C lesion according to the ACC/AHA classification14, in a coronary vessel subtending a myocardial area with significant ischaemia, were enrolled.
Patients undergoing RA were further divided into two subgroups according to clinical presentation: stable angina patients (SAP) or non-ST-elevation myocardial infarction (NSTE-ACS) patients according to the ESC guidelines15. The long-term outcome of the NSTE-ACS population undergoing RA was further compared with NSTE-ACS patients not undergoing RA.
Exclusion criteria were ST-segment elevation myocardial infarction (STEMI) within 30 days, cardiogenic shock, in-stent restenosis and angiographically visible thrombus at the target lesion.
Procedural features and follow-up were previously described16.
DEFINITIONS AND ENDPOINTS
Outcome adjudication was performed by the referring physician. In particular, each endpoint was considered as follows:
1. Angiographic success was defined as residual stenosis less than 30% in the presence of TIMI flow grade 3.
2. Slow-flow/no-flow phenomenon was defined with a semi-quantitative method as delayed or absent distal opacification of the coronary artery on angiography, according to the TIMI flow definition, in the absence of significant coronary artery disease17.
3. Coronary perforation was defined as evidence of extravasation of dye or blood from the coronary artery during or following the interventional procedure18.
4. Periprocedural MI was defined as the elevation of serum creatinine kinase isoenzyme MB or troponin at least five times over the baseline value following the procedure, associated with clinical signs of angina or ECG signs of ischaemia or new echocardiographic hypo-akinesia19. The cut-off values used for troponin were: troponin T 0.03 ng/mL, troponin I 0.04 ng/mL.
5. Stent thrombosis was defined according to the Academic Research Consortium criteria20.
6. Chronic kidney disease (CKD) was defined as kidney damage or glomerular filtration rate <60 mL/min/1.73 m² (according to the MDRD formula) for three months or more, irrespective of cause21.
7. Vasculopathy was considered as any stenotic (>50%), occlusive, or aneurysmal disease of the aorta and its peripheral branch arteries, excluding the coronary arteries.
8. Death was defined as all-cause mortality; all deaths were considered cardiac, unless unequivocally non-cardiac.
The primary endpoint of the study was angiographic success. The secondary endpoint was a composite of procedural complications including procedural perforation, slow flow/no-flow, periprocedural myocardial infarction and in-hospital MACE (sum of death and myocardial infarction). Other key secondary endpoints were death, non-fatal MI and MACE during follow-up.
STATISTICAL ANALYSIS
This has been previously described16. In this sub-analysis, for propensity score, first logistic regression analysis was carried out for all baseline features that differed between provisional and two-stent groups and those features that were clinically relevant (age, gender, diabetes mellitus, previous surgical or percutaneous revascularisation, chronic kidney disease, EF), and matching was computed after division into quintiles and methods of nearest neighbour on the estimated propensity score. Calibration was tested with the Hosmer-Lemeshow test, and accuracy was assessed using area under the curve analysis. A two-sided p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences, Version 18 (SPSS Inc., Chicago, IL, USA).
Results
Our registry included 1,308 patients, 37% (484) in the NSTE-ACS group and 63% (824) in the SAP group. Baseline clinical features are shown in Table 1.
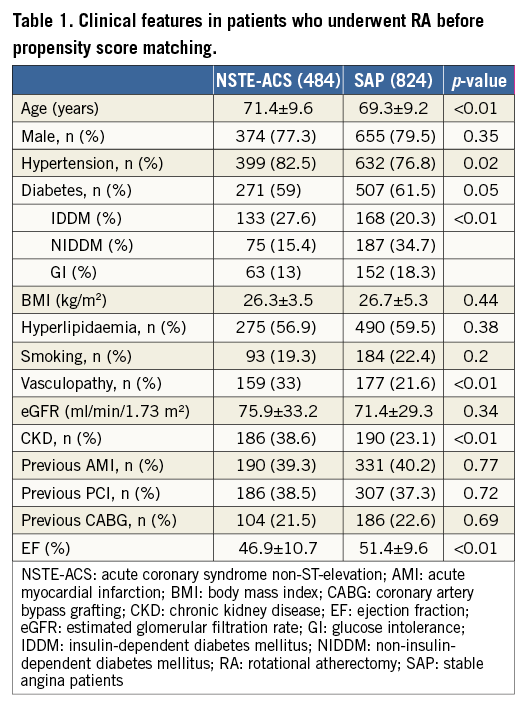
Compared with SAP, patients with NSTE-ACS were older (71.4±9.2 vs. 69.3±9.2 years, p=0.001), had more hypertension (78.9% vs. 76.8%, p=0.019), diabetes mellitus (27.6% vs. 20.3%, p=0.003), peripheral vascular disease (38.6% vs. 23.1%, p<0.001), renal impairment (38.6% vs. 23.1%, p<0.001) and a lower left ventricular ejection fraction (46.9±10.7% vs. 51.4±9.6%, p<0.001).
The indication for RA was elective in about half of the cases in both groups (49.2% vs. 50.9%, p=0.73). Independent predictors for RA in bail-out cases were vasculopathy (HR 2.7, CI: 1.2-6.3), multivessel disease (HR 1.7, CI: 1.4-1.9, p<0.001), bifurcation lesions (HR 1.9, CI: 1.4-2.3, p=0.02) and low TIMI flow (HR 10.5, CI: 8.8-12.5, p=0.009).
Procedural features are shown in Table 2. The target lesions were primarily located in the left anterior descending artery (57% vs. 54%, p=0.301), and mostly treated with second-generation drug-eluting stents (65.3% vs. 62%, p=0.26). The transfemoral approach was the most frequently used in both groups (70.2% vs. 72.3%, p=0.438).
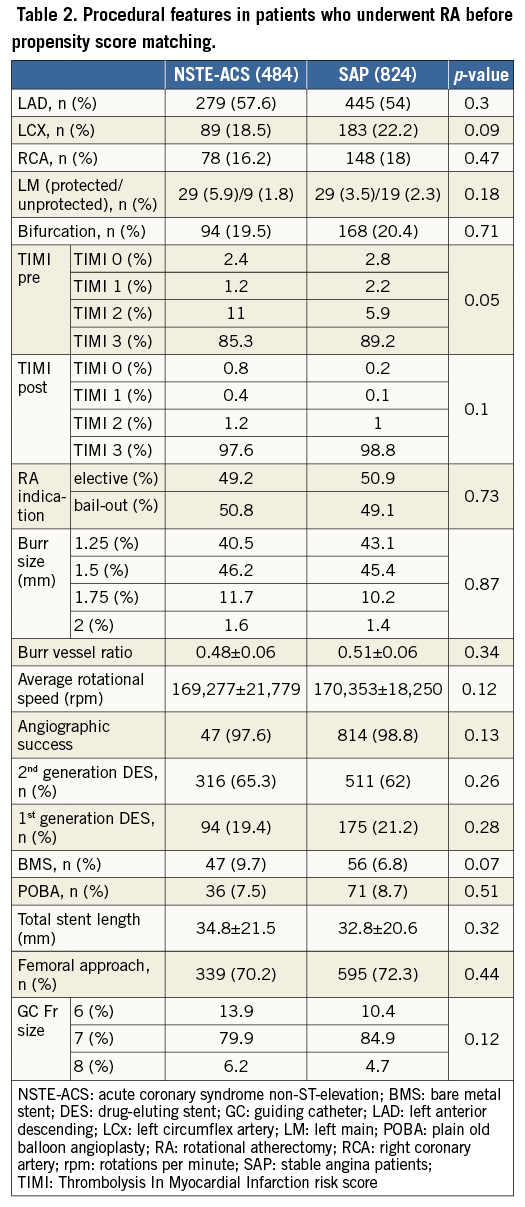
Procedural success was similar in the two groups; mean post-procedure TIMI flow (2.9±0.3 vs. 2.98±0.2, p=0.058) and angiographic success (98.8% vs. 99.2%, p=0.57) were not significantly different (Figure 1).
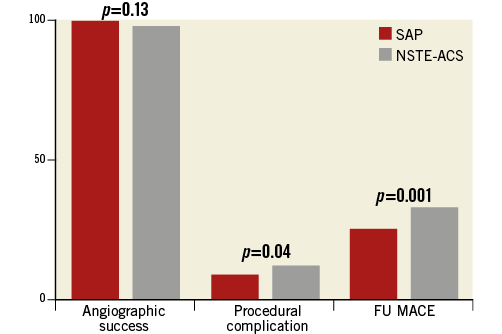
Figure 1. Outcome in patients who underwent RA. MACE: major adverse cardiovascular events; NSTE-ACS: non-ST-elevation acute coronary syndrome; SAP: stable angina patients
Procedural complications at univariate analysis were more frequent in the NSTE-ACS group compared with the SAP group (11.3% vs. 8%, RR 1.42, CI: 1.1-1.99, p=0.043), driven mainly by a higher rate of slow flow/no-flow (3.3% vs. 1.4%, RR 2.27, CI: 1.08-4.75, p=0.029), while in-hospital death and MACE did not differ (respectively, 1.2% vs. 0.3%, p=0.082 and 5.7% vs. 5.8%, p=0.93) (Table 3). At multivariate analysis, age (HR 1.14, CI: 0.89-1.39, p=0.046) and vasculopathy (HR 2.84, CI: 1.11-7.25, p=0.029) were independent predictors of worse outcome, while there was just a trend for NSTE-ACS (HR 2.39, CI: 0.96-5.96, p=0.061) (Figure 2).
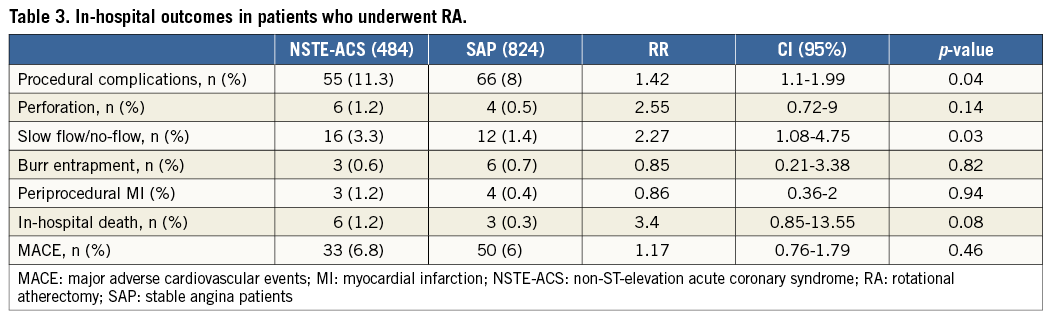
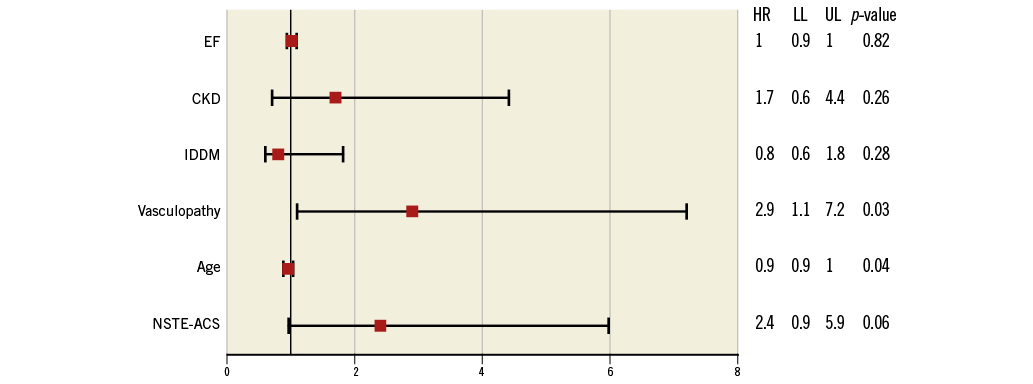
Figure 2. Multivariate analysis for in-hospital MACE. CKD: chronic kidney disease; EF: ejection fraction; HR: hazard ratio; IDDM: insulin-dependent diabetes mellitus; LL: lower limit; NSTE-ACS: non-ST-elevation acute coronary syndrome; UL: upper limit
Follow-up MACE before propensity score matching after a median of 27.9 months (range 9.2-68.7 months) was not different between the two groups in terms of length of follow-up (SAP group 28.5±25 vs. NSTE-ACS 28.1±21 months, p=0.13) or loss to follow-up (17% vs. 19%, p=0.36). The cumulative MACE incidence was significantly higher in the NSTE-ACS group compared with the SAP group (32.4% vs. 24.2%, RR 1.33, CI: 1.12-1.59), and in patients with NSTE-ACS who did not undergo RA (6.8%, log-rank, p=0.001) (Table 4, Figure 1, Figure 3). At multivariate analysis, NSTE-ACS and age were independent predictors of worse outcome (respectively, HR 5.43, CI: 1.01-9.09, p=0.045, and HR 1.7, CI: 1.41-1.98, p=0.033) (Figure 4).
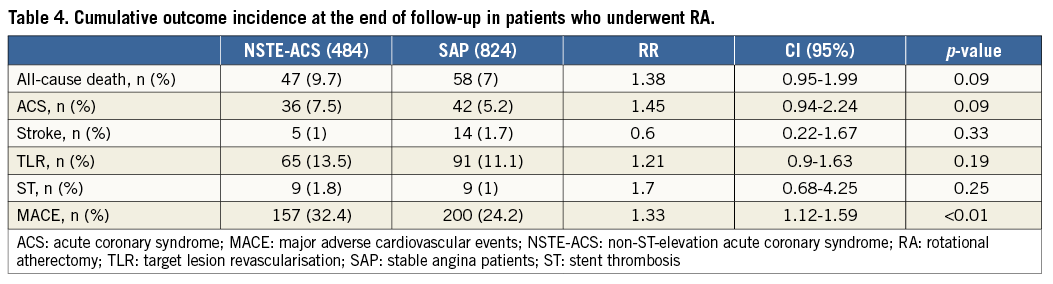
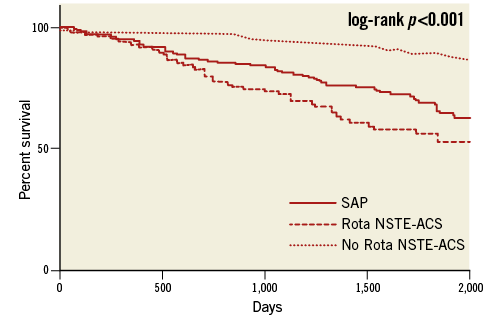
Figure 3. Kaplan-Meier curves before propensity score matching. No Rota NSTE-ACS: non-ST-elevation acute coronary syndrome patients not undergoing rotational atherectomy; Rota NSTE-ACS: non-ST-elevation acute coronary syndrome patients undergoing rotational atherectomy; SAP: stable angina patients undergoing rotational atherectomy
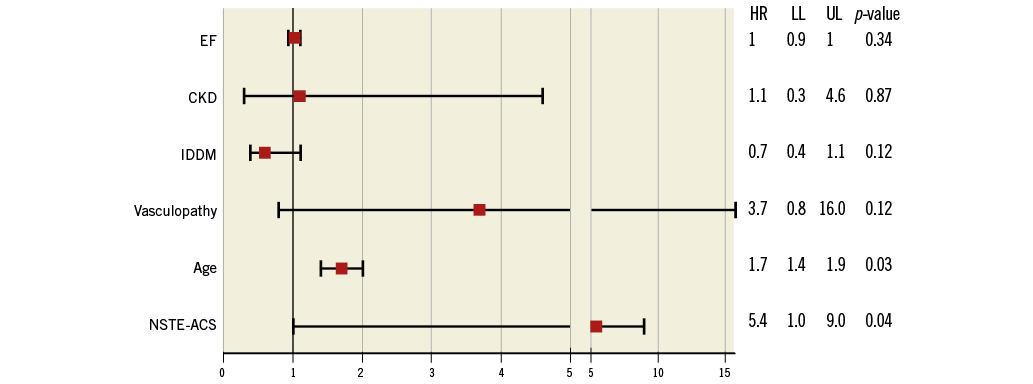
Figure 4. Multivariate analysis for follow-up MACE. CKD: chronic kidney disease; EF: ejection fraction; HR: hazard ratio; IDDM: insulin-dependent diabetes mellitus; LL: lower limit; NSTE-ACS: non-ST-elevation acute coronary syndrome; UL: upper limit
At follow-up after propensity score matching, no differences persisted between patients with NSTE-ACS who did or who did not undergo RA in terms of MACE (16% vs. 13%, log-rank p=0.14) (Table 5, Figure 5).
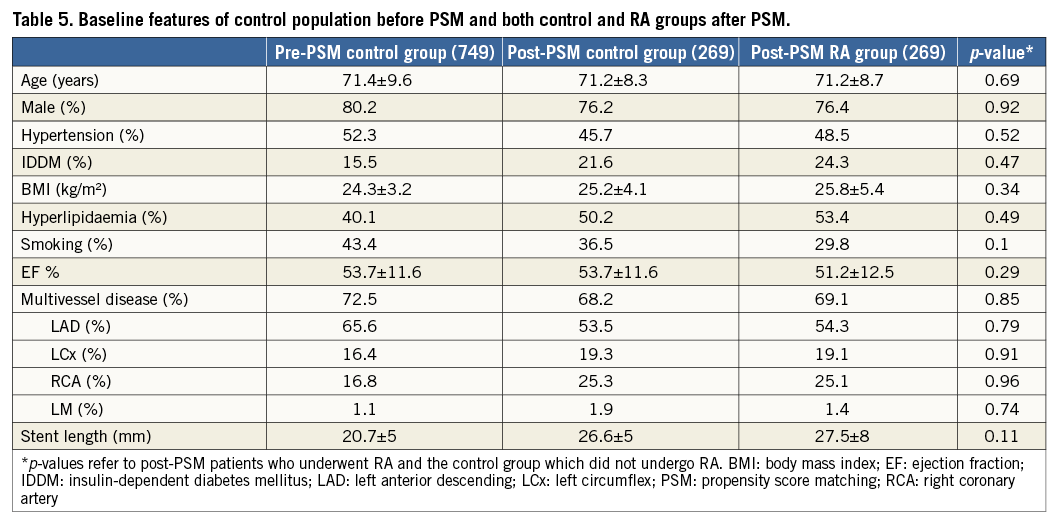
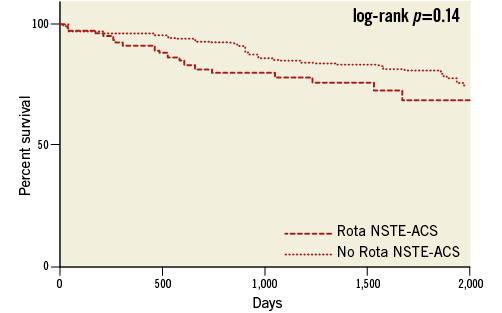
Figure 5. Kaplan-Meier curves after propensity score matching. No Rota NSTE-ACS: non-ST-elevation acute coronary syndrome patients not undergoing rotational atherectomy; Rota NSTE-ACS: non-ST-elevation acute coronary syndrome patients undergoing rotational atherectomy; SAP: stable angina patients undergoing rotational atherectomy
Discussion
The main findings of our study were:
1) RA has similar procedural success in patients with NSTE-ACS and SAP.
2) NSTE-ACS patients experienced more frequent intraprocedural complications than SAP; however, this was mainly driven by older age and vasculopathy, while there was just a trend for the presence of ACS.
3) Patients with NSTE-ACS who underwent RA showed a similar MACE rate compared with a matched population of NSTE-ACS patients who did not undergo RA, comparable with other reports from the literature13.
The ERBAC study22 was the first trial which demonstrated higher procedural success with RA compared to POBA in calcific lesions, although with a higher risk of subsequent revascularisation. Comparable data were shown in the DART trial23, which demonstrated a procedural success of 91.6% with a complication rate of 5.3%, mainly driven by periprocedural myocardial infarction (2.2%). Although RA is old technology, it continues to raise interest among the interventional cardiology community. Recently, due to the lack of a standard protocol, a European expert consensus proposed some advice for operators24. The lower rates of complications in the present registry and the higher procedural success are due to the improvement of techniques, materials (such as second-generation stents), and the utilisation of a smaller burr to vessel ratio (in our study 0.5) with a pecking motion in accordance with European expert consensus24. A more recent paper by Benezet et al25 shows a comparable angiographic success compared to our data.
Of interest, in our registry there were no differences in RA indication between the two groups. RA was used as elective in about half of the cases in both groups, indicating that operators felt comfortable with the procedure, while clinical and angiographic characteristics, such as low TIMI flow and bifurcation lesions, seem to predict its employment in bail-out.
On the other hand, while NSTE-ACS patients more frequently showed procedural complications, in-hospital MACE were similar. This finding could be explained by a worse clinical condition in this subset of patients with coronary calcified lesions requiring RA compared to patients who daily undergo coronary angioplasty for SA. Interestingly, acute coronary syndrome itself was not a strong independent predictor for complications, but showed just a trend for statistical significance. Moreover, we reported nine cases (0.7%) of burr entrapment, in line with previous reports26.
Finally, patients with NSTE-ACS showed a higher rate of MACE during follow-up compared with SA patients; however, when compared with a matched population with NSTE-ACS, this difference no longer persisted.
Mezilis et al reported a MACE rate of 11.3% during three years of follow-up after RA27. Abdel-Wahab et al28, in a cohort of 255 patients who underwent DES implantations after RA, reported a MACE rate of 17.7%, a death rate of 4.4% and a TVR rate of 9.9% during a follow-up of 15 months. Furthermore, in the ROTALINK I study registry29, after a mean follow-up of 28.4 months, the overall MACE rate was 19.8%, mainly driven by target lesion revascularisation (11.7%).
The worse outcome of the NSTE-ACS group is partially related to RA itself, while the worse prognosis of acute coronary syndrome itself also impacts on these patients, as demonstrated by the results derived from the propensity score matching. Comparable data are present in the literature: Roe et al30, in an elderly population (>65 years old) with NSTE-ACS, after five years of follow-up, reported a long-term mortality rate of 33.5% while the MACE rate was 44.9%. Similar data were also reported in the statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology in collaboration with the Society of Geriatric Cardiology31.
Limitations
The main limitation of this study was the observational design and the absence of a central independent angiographic core lab for the endpoint definition. Second, patient selection for RA was not centralised but was at each centre’s discretion. A selection bias regarding patients with ACS and a low thrombotic burden could not be excluded. Third, we do not have complete data about medical therapy. Fourth, about 20% of patients, reflecting the timing of their enrolment, were treated with first-generation DES. Finally, the incidence of periprocedural MI was lower than that reported in other similar trials, due to the absence of a central committee adjudicating events and to the lack of routine collection of post-procedural troponin levels in all centres.
Conclusions
Rotational atherectomy has a similar procedural success and safety profile in patients with NSTE-ACS compared with SAP. The higher rate of adverse cardiac events at follow-up in NSTE-ACS patients undergoing RA is comparable with a matched population of NSTE-ACS patients not undergoing RA. A prospective randomised trial is warranted.
| Impact on daily practice Despite the fact that it is generally contraindicated, rotational atherectomy (RA) could be a safe and efficacious procedure even in patients with NSTE-ACS. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

