Abstract
Background: Calcified coronary lesions present therapeutic challenges for the interventional cardiologist, often requiring rotational atherectomy (RA).
Aims: This study aimed to develop an angiographic scoring tool to predict the need for a priori RA.
Methods: A pooled analysis of the randomised ROTAXUS and PREPARE-CALC studies was carried out, (N=220 patients, N=313 lesions), by virtue of the fact that both studies made provision for crossover to RA (from balloon dilatation or modified balloon dilatation, respectively). Logistical regression techniques were employed to assess for the presence of patient- or lesion-specific factors leading to a necessity for RA. External validation was performed though retrospective calculation of the score for 192 patients who underwent bail-out RA in a single centre.
Results: Lesion length (odds ratio [OR] 1.02, 95% confidence interval [CI]: 1.00-1.04 per mm, p=0.04), bifurcation lesion (OR 2.60, 95% CI: 1.27-5.30, p=0.009), vessel tortuosity >45° (OR 3.49, 95% CI: 1.73-7.03, p<0.001) and severe vessel calcification (OR 11.60, 95% CI: 3.40-39.64, p<0.001) were predictive of the need for RA in multivariate analysis. Based on the regression coefficients, a scoring system was devised. The greater the score, the more likely a lesion required RA. The scoring system performed well in the external validation cohort, with 78% of patients crossing over having a score of greater than the proposed cut-off of 3.
Conclusions: We provide an angiographic scoring tool to support the expeditious use of time and resources, allowing assessment of the likelihood of success of a balloon-based strategy, or the necessity for RA.
Introduction
With advancing age and increase in patient comorbidities, particularly diabetes mellitus and end-stage renal failure, comes a significant burden of complex calcific coronary disease, i.e., lesions with a combination of the following factors which are known to increase the difficulty of percutaneous coronary intervention (PCI) – tortuosity, calcification and bifurcations. Rotational atherectomy (RA) remains the most widely available bail-out device in calcified coronary lesions that cannot be crossed or adequately dilated with a balloon. Its mechanism of action is the removal of obstructive atheroma by differential cutting1. To date, no randomised study has yielded outcome data highlighting those (patient or lesion) characteristics which advocate a priori RA rather than a provisional strategy.
Given that almost 50% of patients in the ROTATE registry had RA performed as a bail-out strategy, and similarly almost 40% in a second single-centre registry, identifying characteristics which more clearly define an appropriate subset of patients for a priori RA has the potential advantages of reduced procedural time, contrast agent dose, radiation and cost, not only in the sense of avoiding a failed attempt at balloon-only techniques in those patients who require RA, but also in allowing one to identify those patients where the additional effort of RA is not necessary23. Therefore, we sought to investigate whether there are particular patient or lesion characteristics which are predictive of the need for RA, utilising data from the Rotational Atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease (ROTAXUS) and the Comparisons of Strategies to Prepare Severely Calcified Coronary Lesions (PREPARE-CALC) studies45.
Methods
STUDY DESIGN
The present analysis is based on data from two randomised trials that compared lesion preparation with balloon predilatation versus RA in patients with calcified coronary artery disease undergoing PCI with drug-eluting stents (DES). Individual patient-level data were pooled into a common database at the Heart Center Leipzig at University of Leipzig (Leipzig, Germany). Each study was approved by the ethics committee at each participating centre, and all patients signed written informed consent.
The individual study design, definitions, and endpoints used within both studies and trial methodology have been described previously45. A brief summary is presented here.
ROTAXUS
The ROTAXUS study (NCT00380809) was performed by randomising 240 patients with moderate or severe coronary calcification 1:1 to a strategy of standard balloon predilatation or RA followed by stenting. All patients had documented myocardial ischaemia and complex calcified coronary artery lesions. The trial was performed at three high-volume, experienced interventional study sites in Germany. Stenting was performed using the paclitaxel-eluting TAXUS Liberté stent (Boston Scientific). Rotablator (Boston Scientific) burr size was selected to reach a burr/vessel ratio of 0.5-0.7 and RA speed ranged between 140,000 and 180,000 rotations per minute. The primary endpoint of the trial was in-stent late lumen loss (LLL) at nine months, defined as the difference between the immediate post-procedure in-stent minimal luminal diameter, and the in-stent minimal lumen diameter at nine-month follow-up angiography.
PREPARE-CALC
The PREPARE-CALC study (NCT02502851) was performed by randomising 200 patients with severe coronary calcification 1:1 to a strategy of coronary lesion preparation using modified balloons (MB), cutting or scoring, or RA followed by DES implantation. All patients had documented myocardial ischaemia and severe calcification of the target lesion as defined by cineangiography. The trial was performed at two high-volume, experienced interventional study sites in Germany. Optical coherence tomography (OCT) was recommended before lesion preparation and at the end of the procedure, but was not used to guide clinical decision making. Stenting was performed using a new-generation sirolimus-eluting stent with a bioabsorbable polymer (Orsiro; Biotronik AG). The primary endpoint was strategy success, defined as successful stent delivery and expansion with attainment of <20% in-stent residual stenosis of the target lesion in the presence of Thrombolysis In Myocardial Infarction (TIMI)3 flow without crossing over to the RA group or stent failure. In-stent LLL was also measured as a co-primary endpoint.
CURRENT ANALYSIS
Both trials allowed crossover to RA in the balloon group using pre-specified criteria, specifically that the lesion was either not crossable by any balloon, not adequately dilatable with a balloon, or if there was a failure of stent delivery. Thus, by the designs of both trials, it was possible to perform a comparative post hoc analysis of patients who did and did not require bail-out RA in the balloon group.
The study flow chart is summarised in Figure 1. All patients in the original ROTAXUS and PREPARE-CALC trials who had been randomised to either standard or modified balloon predilatation were assessed for inclusion in this study (pooled data set). A single patient in this group was excluded from the present analysis as the crossover status was unknown. The remaining patients were then split into two distinct groups, those in whom PCI was accomplished without the need for RA (the angioplasty only group), and those in whom PCI was only possible with bail-out RA (the bail-out RA group).
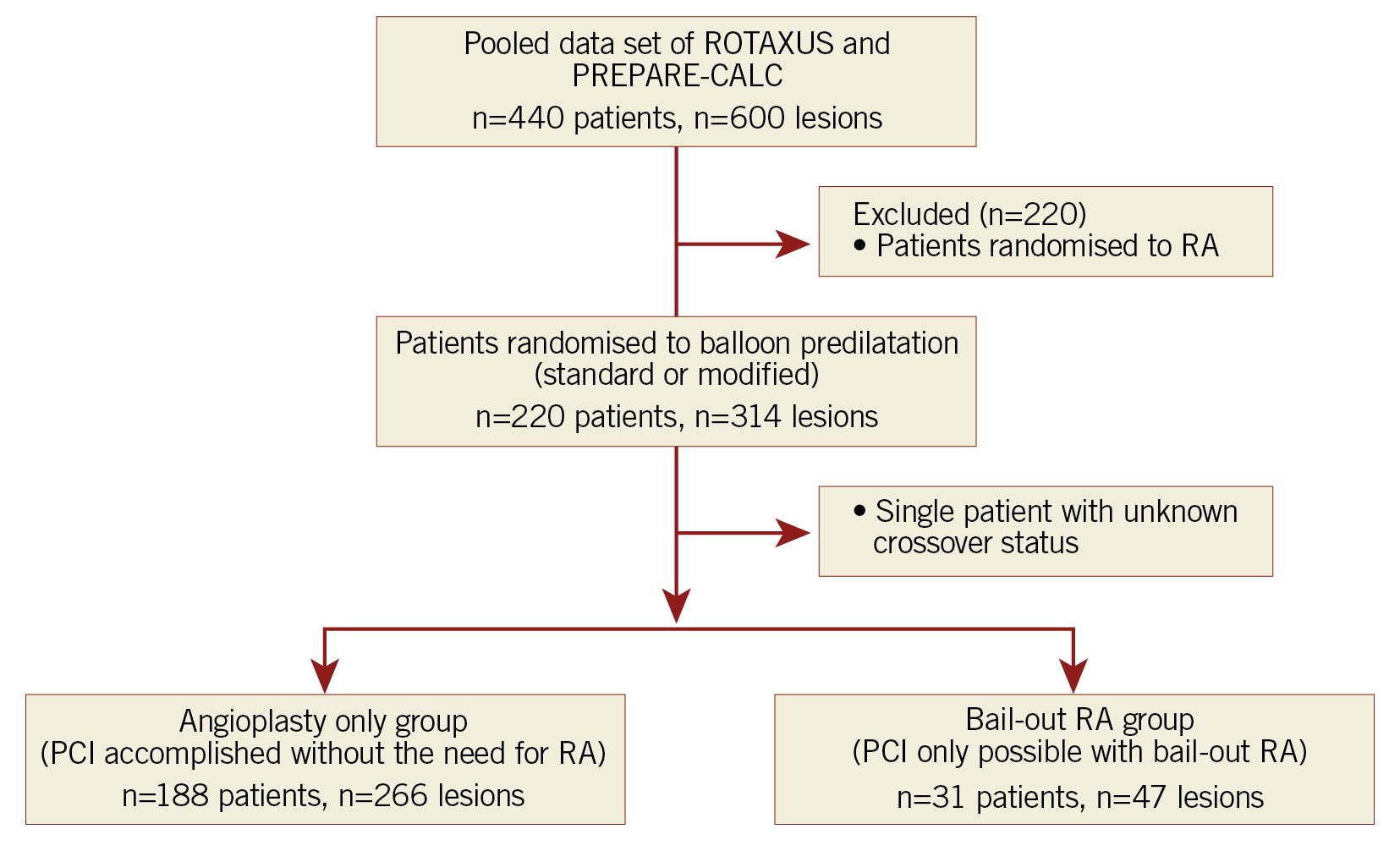
Figure 1. Study flow chart. PCI: percutaneous coronary intervention; PREPARE-CALC: Comparison of Strategies to Prepare Severely Calcified Coronary Lesions; RA: rotational atherectomy; ROTAXUS: Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease
Specifically, we assessed whether there was a statistically significant difference in the baseline clinical demographics and lesion characteristics of the angioplasty only group and the bail-out RA group. Calcification was core lab adjudicated, and defined as severe if, at cardiac catheterisation, radiopacities were noted without cardiac motion before contrast injection generally compromising both sides of the arterial lumen. Procedural outcomes assessed were duration, fluoroscopy time, contrast amount, stent loss, perforation, pericardial effusion and angiographic success. In-hospital clinical outcomes consisted of death, myocardial infarction (MI), target vessel revascularisation (TVR) and stent thrombosis. Clinical outcomes assessed at nine months were death, MI, TVR and target lesion revascularisation (TLR), obtained through data collected via clinical follow-up and scheduled repeat coronary angiogram.
STATISTICAL ANALYSIS
Continuous data are presented as mean±SD. Categorical data are presented as counts and proportions (%). For patient-level data, differences between groups were checked for significance with the Student’s t-test or Wilcoxon rank-sum test (continuous data) or the chi-square or Fisher’s exact test, as required. For lesion-level data, differences between groups were checked for significance with general estimating equations to address intrapatient correlation in patients who underwent multilesion intervention. Variables with p-values <0.2 in unadjusted analyses were considered for inclusion in the multivariate model6. Confidence intervals for the odds ratios (OR) were constructed based on a logarithmic transformation. All tests were two-sided, and a p-value of <0.05 was considered statistically significant.
The method described by Moons et al7, was used to develop a scoring rule, based on the regression coefficients obtained in multivariate binomial logistic regression analysis, to predict which patients will fail balloon dilatation and require RA. In brief, the sum of the regression coefficients of the predictors of interest, rounded to the nearest integer, was used as the score. Lesion length was dichotomised, choosing 20 mm as a cut-off, as this represents both the median value of lesion length in our study, and the cut-off for an American Heart Association/American College of Cardiology (AHA/ACC) type C lesion8. Receiver operating characteristic (ROC) analysis was performed to assess discriminative power. A Hosmer-Lemeshow goodness of fit test was performed to assess calibration.
The pooled data set was used as an internal validation cohort, excluding two patients for whom values of all parameters of interest were not available. For each risk score, the predicted probability of crossover was calculated using the method described by Sullivan et al9. Calibration of the score was assessed in the internal validation cohort by dividing the sample into five equal groups based on the predicted probability, and plotting the mean probability of each quintile against the proportion of observed crossovers to RA for that quintile. A sensitivity analysis, outlined in Supplementary Appendix 1, was performed to compare the performance of the scoring system to that of the logistic regression model itself.
To assess performance in an external population, retrospective calculation of the score for 192 patients who were known to have undergone bail-out RA in a single centre (Bad Segeberg, including some of the patients previously described in the paper by Allali et al3 but with more recent cases added) was performed.
All statistical analysis was carried out in SPSS (IBM SPSS Statistics Subscription, Build 1.0.0.1508; IBM). The authors had full access to all data in the study and take responsibility for its integrity and that of the data analysis.
Results
BASELINE CLINICAL AND ANGIOGRAPHIC CHARACTERISTICS
Of the pooled data set consisting of the patient population enrolled in the ROTAXUS and PREPARE-CALC studies, a total of 220 patients were randomised to balloon predilatation. Of these, a total of 31 patients required bail-out RA. Essentially, there were no significant differences in any of the patient characteristics across groups (Supplementary Table 1). Multivessel disease was present in the majority of patients in both groups.
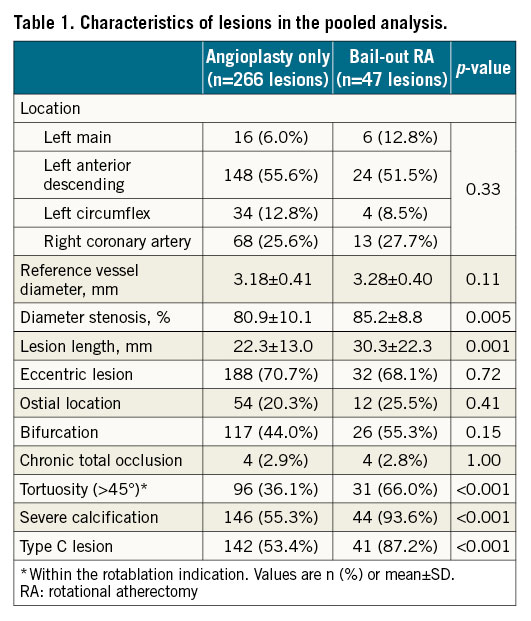
Overall, 313 lesions were treated (1.43 lesions per patient), 266 in the angioplasty only group and 47 in the bail-out RA group. The target lesions were high grade stenoses, with a mean diameter of stenosis by visual estimate of 80.9±10.1% in the angioplasty only group, and of 85.2%±8.8% in the bail-out RA group. Lesion characteristics differed in a statistically significant manner between the angioplasty only and the bail-out RA groups (Table 1). Mean stented length in the balloon angioplasty group was 29.10±15.79 mm, and in the bail-out RA group 30.98±15.54 mm. Mean stent size in the balloon angioplasty group was 3.2±0.4 mm, and in the bail-out RA group 3.3±0.4 mm. In the balloon angioplasty group 44% of cases involved a bifurcation lesion, and in the bail-out RA group 55% of cases.
CAUSES OF CROSSOVER TO RA
The most common reason for crossing over to RA was that the lesion was unable to be crossed by any balloon (48.9%). In 31.9% of cases, the lesion was not adequately dilatable, and in 17.0% of cases, it was not possible to deliver any stent. Finally, in 2.2% of cases, RA was introduced as a bail-out in the case of an underexpanded stent (Supplementary Table 2). The percentage crossover (on a per-patient basis) in ROTAXUS was 12.5%, and in PREPARE-CALC 16%, with a total crossover percentage of 14%.
PROCEDURAL OUTCOMES
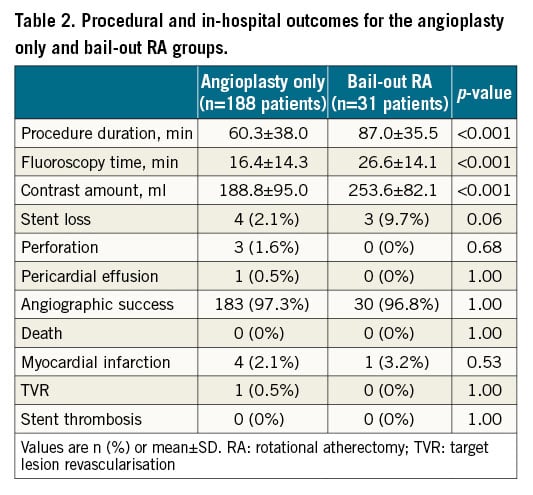
Procedural complications and outcomes are shown in Table 2 and Supplementary Table 3, respectively. A significant difference in procedural duration, fluoroscopy time and contrast volume utilised was noted in the bail-out RA group compared to the angioplasty only group (Table 2). Supplementary Table 3 provides a comparison of the outcomes of the bail-out RA group to those of the elective RA group, revealing a statistically significant difference in contrast agent dose and stent loss, with greater stent loss in the bail-out versus elective RA group (3 cases [9.7%] vs 1 case [0.5%] respectively, p<0.007).
NINE-MONTH CLINICAL OUTCOMES
Nine-month clinical outcomes are shown in Figure 2. However, in summary they show no statistically significant difference in all-cause death, MI, TVR or TLR.
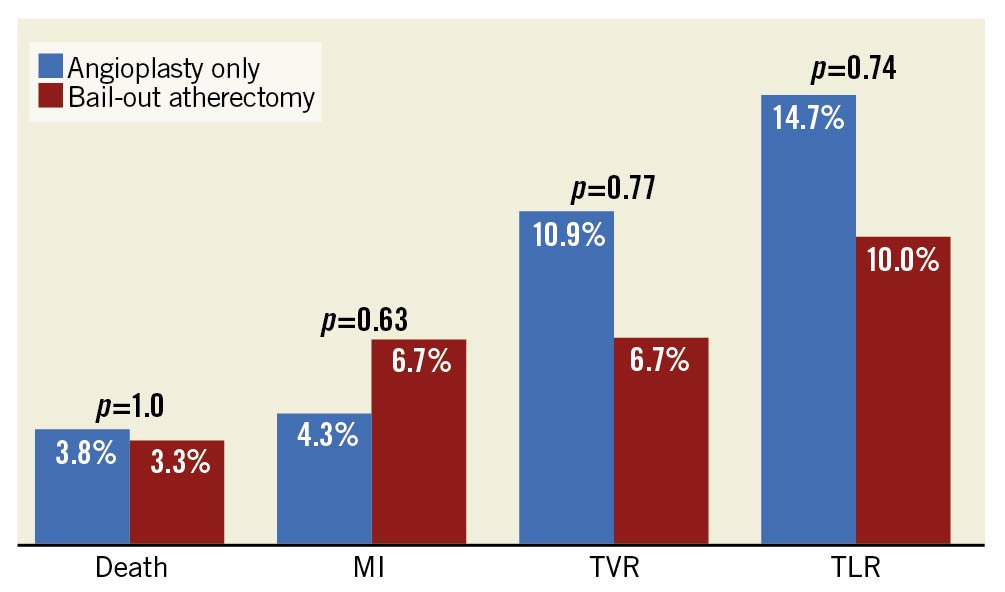
Figure 2. Clinical outcomes at nine months post intervention, stratified by intervention strategy. MI: myocardial infarction; TLR: target lesion revascularisation; TVR: target vessel revascularisation.
PREDICTORS OF BAIL-OUT ATHERECTOMY
Lesion characteristics with a p-value <0.2 in univariate analysis (specifically reference vessel diameter, lesion length, diameter stenosis, bifurcation lesion, severe calcification, and tortuosity >45°) were entered into a multivariate logistical regression model. ACC lesion classification was not included in the logistical regression analysis due to the significant overlap in this classification with the other independent variables. Predictors of the need for bail-out RA identified on multivariate analysis were lesion length (OR 1.02 per mm, 95% CI: 1.00-1.04, p=0.04), bifurcation lesion (OR 2.60, 95% CI: 1.27-5.30, p=0.01), severe calcification (OR 11.60, 95% CI: 3.40-39.64, p<0.001) and tortuosity >45° (OR 3.49, 95% CI: 1.73-7.03, p<0.001) (Figure 3, Supplementary Table 4). The logistic regression model fits the data well in terms of discrimination and calibration, with a C-statistic of 0.80 (95% CI: 0.75-0.87) and a Hosmer-Lemeshow goodness of fit test with a p-value of 0.385 for χ2 (8)=8.508.
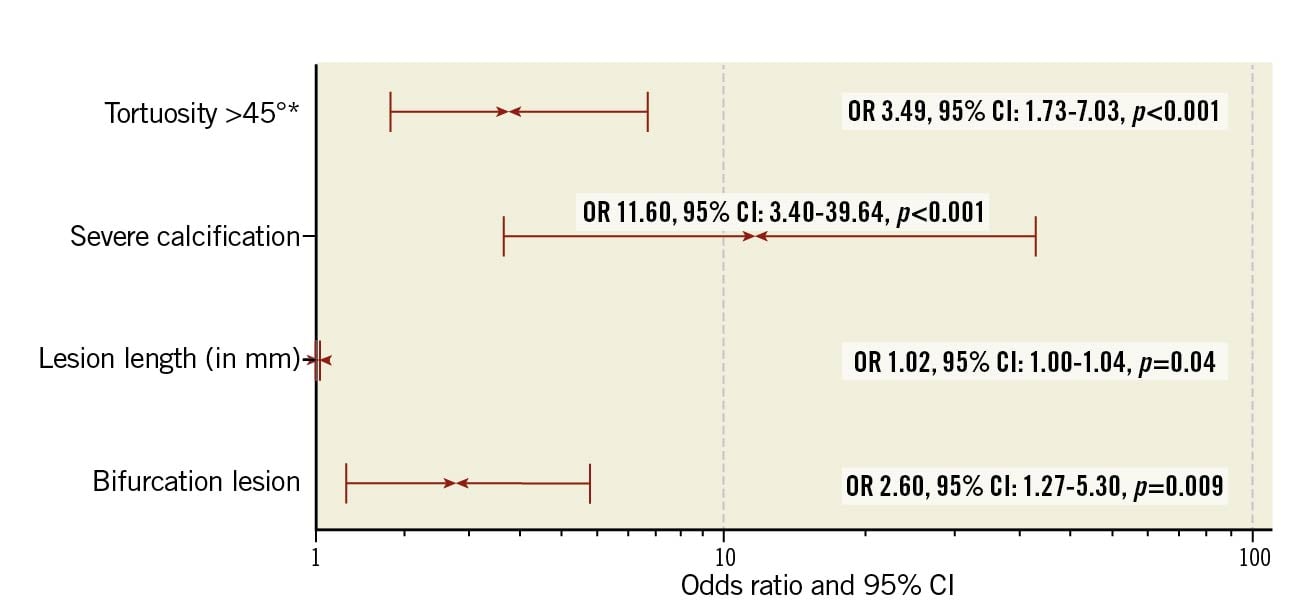
Figure 3. Predictors of bail-out atherectomy, as obtained through binominal logistic regression analysis. * Within the rotablation indication. CI: confidence interval; OR: odds ratio
SCORING RULES (“ROTASCORE”)
The scoring system obtained from the multivariate analysis is outlined in the Central illustration. In the scoring system, a value of 0.5 was assigned to a lesion length >20 mm, a value of 1 to the presence of a bifurcation lesion or tortuosity of an artery segment >45°, and a value of 2 to the presence of severe calcification. The greater the overall score, the more likely a lesion required an RA strategy. Internal validation analysis via C-statistics (Central illustration) revealed good discrimination, with an AUC of 0.79 (95% CI: 0.73-0.85). A cut-off of score of 3 correctly identified 79% of cases where RA was required, albeit at a cost of selecting 34% of cases where it was not necessary. The accuracy of the scoring system is graphically represented in the Central illustration. Further specifics with regard to the calibration of the scoring system are outlined in Supplementary Appendix 2 and Supplementary Figure 1-Supplementary Figure 3.
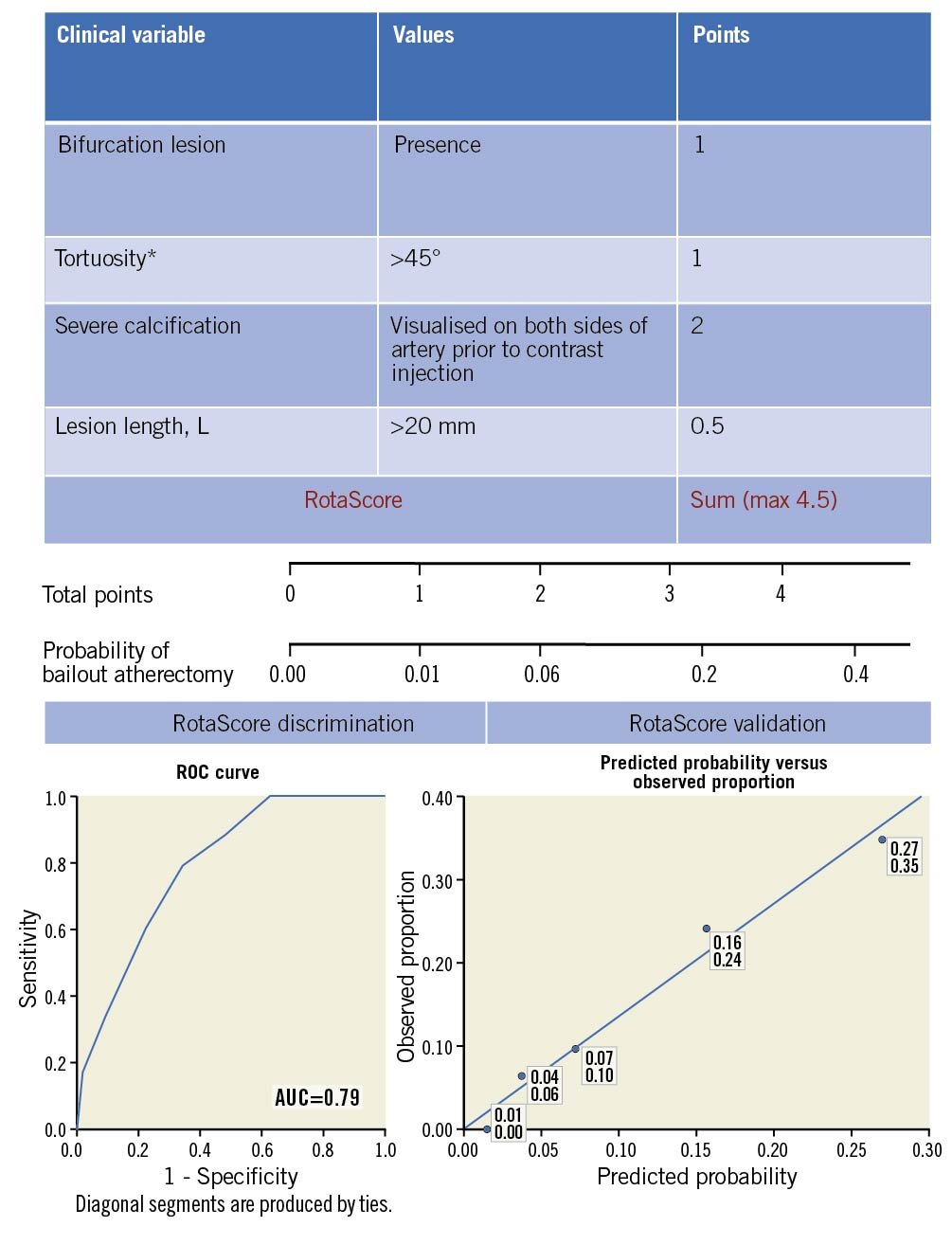
Central illustration. Scoring system derived from post hoc analysis of ROTAXUS and PREPARE-CALC data to predict the probability of requiring rotational atherectomy to achieve angiographic success. Top: outline of the components of the scoring system and their relative weights, derived from the regression coefficients obtained in multivariate binomial regression analysis. * Within the rotablation indication. Middle: scaled representation of change in probability of requiring bail-out atherectomy as a function of RotaScore. Bottom, left panel: receiver operating characteristic curve for the RotaScore. The area under the curve (AUC) is 0.79 (95% CI: 0.73-0.85). Bottom, right panel: calibration of the RotaScore was assessed by dividing the combined cohort, less two lesions for which missing data prevented a total RotaScore from being calculated (n=311 lesions), into five equal groups per the predicted probability of requiring crossover to a rotational atherectomy strategy. Subsequently, the mean probability of crossover for each group was plotted against the observed proportion of same. The upper number denotes the mean predicted probability, the lower the average observed crossover proportion for a particular quintile.
EXTERNAL VALIDATION
The patient characteristics of the external validation group are outlined in Supplementary Table 5, and lesion characteristics are outlined in Table 3. Both patient and lesion characteristics are similar to those of the derivation cohort.
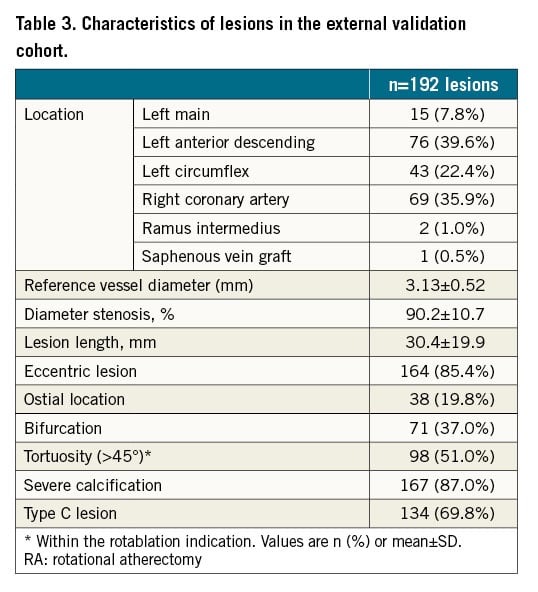
For the external validation group, mean procedure time was 117.6 mins, fluoroscopy time 42.9 mins and mean contrast volume used was 281.2 ml. In 35% of cases, RA was required as the lesions were uncrossable. In 46% of cases, lesions were undilatable and in 19% of cases, it was not possible to deliver a stent despite the lesion having been crossed and predilated. A mean of 2.2 stents were inserted per case, and average stent length was 49.7±26.7 mm.
The average RotaScore was calculated for this external cohort and compared to those who did and did not require bail-out atherectomy in the internal (derivation) cohort. In this external validation cohort, mean RotaScore was 3.2±1.2, with 78% of patients having a score of 3 or greater. This compares to a mean RotaScore of 3.1±0.7 for those patients who crossed over versus a mean RotaScore of 1.9±1.2 for those patients who did not cross over in the derivation cohort, with more than 79% of patients crossing over having a RotaScore of 3 or more (p<0.0001 for the comparison of means in the internal validation cohort).
Discussion
In this study, the following lesion characteristics, combined in the RotaScore, were shown to be predictors of the need for an RA strategy – lesion length, bifurcation lesion, severe calcification and tortuosity >45°. There were no patient-level characteristics which predicted the need for atherectomy.
The presence of severe calcification was found to be most significant in terms of predicting the need for RA. Severe coronary calcification may be encountered in up to 20% of patients treated with PCI10. PCI in those patients with calcified coronary lesions is usually more challenging, and the risk of complications has been shown to be higher if appropriate consideration is not given to the planning and execution of the procedure10. Thus, attention to those factors which predict the likely need for an RA strategy, summarised in our RotaScore, can ensure most expeditious use of time and resources by allowing the operator to make an initial a priori decision to use RA, rather than first attempting balloon dilatation and then being forced to switch to RA after this fails, increasing procedure time, cost, radiation and contrast agent dose. The scoring system identified in this study can facilitate the avoidance of “rota regret”11, in terms of allowing the operator to identify more accurately the situations where an initial plan for RA is a superior strategy. It also has the advantage of helping to prevent a situation whereby it is necessary to use RA in retrospect through an underexpanded, undilatable stent, which has been shown to lead to a significantly increased risk of complications1213.
Although previous studies have attempted to identify those patient and lesion characteristics predictive of outcomes in RA, they have not had the benefit of a data set derived from randomised controlled trials. The ROTATE multicentre registry2, assessed 1,076 patients with calcified coronary lesions treated by RA and, on multivariate analysis, the presence of type C lesion was found to be an independent predictor of in-hospital major adverse cardiac events. However, the issue with registry data such as these is that there is no comparison control group. Although 30% of RA cases were carried out due to device failure, and imaging guidance was used in 5.1%, an upfront decision was made to perform RA in 37.3% of cases. Furthermore, in 27.6% of cases, the rationale behind the decision to perform RA was not available. Additional retrospective studies were identified, which attempted to look at patient characteristics and outcomes in a group of patients who underwent upfront RA versus bail-out RA; however, these studies were not randomised314. The key advantage of the RotaScore is that it is unique in being derived from a patient cohort that is made up exclusively of patients with highly complex calcific disease, who were randomised to treatment with balloon dilatation or RA, and had provision made for crossover in the event of strategy failure. To our knowledge, this approach has not previously been addressed in the literature.
The presence of a bifurcation lesion, although previously not shown to be predictive of the need for RA, has been associated with better outcomes when an RA strategy is pursued15. Given that in our patient cohort, in the majority of cases, plaque modification was performed in the main vessel rather than a side branch, we speculate that this may be due to the manner in which RA modifies the lesion substrate (differential cutting), in comparison to treatment with balloons, which allows modification of significant calcium without the carina- or plaque-shifting effect of balloons.
Although the authors used a tortuosity cut-off of 45° for this study, in fact, of the 313 lesions, 186 were adjudicated to have tortuosity of 0-45° (mild), 109 between 45-90° (moderate) and only 18 greater than 90° (severe). When the analysis is run with moderate and severe tortuosity as separate variables, it is only moderate tortuosity that remains significant, which interestingly is in consonance with the fact that severe tortuosity is often viewed as a relative contraindication to RA. Taken together, this suggests that a tortuosity of 45-90° may be the range in which it is most appropriate to consider a priori RA.
In the PREPARE-CALC trial, OCT was recommended before lesion preparation and at the end of the procedure. Although preprocedural OCT results were not used for clinical decision making, they have been analysed separately16. While the authors are aware of existing OCT-based scoring systems to predict stent-expansion, e.g., the Fujino-Mintz criteria17, decision making based on OCT-derived lesion characteristics is significantly limited by the fact that in many cases it was not possible to cross the lesion with the OCT catheter. In the PREPARE-CALC OCT subgroup, in only 6 out of 16 cases requiring bail-out RA was it possible to cross the lesion and image with OCT. This highlights the importance of the use of traditional angiographic characteristics, as summarised by the RotaScore, in terms of assessing lesion suitability for balloon dilatation versus RA. Overall, our criteria have a similar sensitivity in predicting the need for bail-out RA to the Fujino-Mintz criteria for predicting stent underexpansion (AUC 0.79 vs 0.86, respectively) despite the additional information which can be gleaned from OCT where possible17.
Limitations
This study is not without limitations. The most significant limitation of this scoring tool is the lack of a control group in the external validation cohort, that is a group of patients with highly complex calcified disease who were treated with balloon dilatation only. The reason for this limitation is that the ROTAXUS and PREPARE-CALC trials are, to the best of our knowledge, the only two clinical studies in existence, without additional criteria limiting generalisability, which randomised patients to an RA or balloon-based treatment strategy in the DES era. Thus, we do not have access to another randomised cohort, with a suitable control group, in which to validate the score. In addition, despite the protocol-defined criteria in both trials for crossover from the balloon angioplasty to the RA group, ultimately, the final decision to use RA was left to the operator. Given that the trials were conducted in high-volume centres very experienced in RA, results achieved and the point at which bail-out RA is attempted may differ in routine clinical practice.
Furthermore, this is a post hoc analysis of two randomised controlled trials performed during different time periods. The ROTAXUS trial randomised patients during the period August 2006 to March 2010, during which period stenting was performed using first-generation paclitaxel-eluting stents. Thus, some of the devices used in this trial may no longer be reflective of current clinical practice. No invasive imaging was used to guide the treatment approach in the ROTAXUS study. In contrast, patients enrolled in the PREPARE-CALC trial were randomised during the period September 2014 to October 2017, with stenting performed using new-generation sirolimus-eluting stents with a bioabsorbable polymer (Orsiro).
This study is also limited by the relatively small size of the bail-out RA group (31 patients in the bail-out RA group versus 188 patients in the angioplasty only group). Thus, the absolute number of patients used to derive the scoring tool is relatively modest. In particular, this means that few significant differences in clinical outcomes, rather than procedural technical outcomes such as time, radiation and contrast dose, were noted. The exception here is stent loss, which was significantly increased in the bail-out RA group compared to elective RA and, interestingly, contrary to what might be expected with the development of stent technology in the interval between the ROTAXUS and PREPARE-CALC trials, consisted entirely of cases involving Orsiro second-generation sirolimus-eluting stents.
Conclusions
This study revealed for the first time that four lesion characteristics, specifically lesion length, bifurcation lesion, severe calcification and tortuosity >45° are predictive of the need for a priori RA. Using the respective weighting of each individual lesion factor from multivariate analysis, this allowed development of a scoring tool designed to predict upfront the need for, and equally the likelihood of being able to avoid, an RA strategy.
Impact on daily practice
RA is often required to treat complex, heavily calcified lesions and, in combination with drug-eluting stents, has been shown to provide effective and durable results. The decision to perform RA is not often taken a priori, but rather in the event of failure of a balloon dilation strategy, leading to increased procedural and screening times, and contrast dose. To determine which cases are most likely to require an RA approach, the current study identified four factors – lesion length, tortuosity >45°, heavy calcification and the presence of a bifurcation lesion, which predict an increased likelihood of needing an RA strategy. These were combined in a simple, practical scoring system allowing effective discrimination between cases with a low and high probability of needing RA.
Conflict of interest statement
M. Abdel-Wahab declares that his hospital receives speakers’ honoraria and/or consulting fees on his behalf from Medtronic and Boston Scientific. A. Allali is consultant and proctor for Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

