Abstract
Background: Endovascular atherectomy enables minimally invasive plaque removal in peripheral artery disease (PAD).
Aims: We aimed to evaluate the safety and the long-term effectiveness of the Phoenix atherectomy for the treatment of complex and calcified lesions in PAD patients.
Methods: Consecutive all-comer patients with PAD underwent the Phoenix atherectomy. Device safety in terms of perforation and distal embolisation were evaluated. Lesion calcifications were categorised by the Peripheral Arterial Calcium Scoring System (PACSS) and lesion complexity was assessed by the Transatlantic Inter-Society Consensus (TASC). Clinically driven target lesion revascularisation (TLR) was assessed.
Results: A total of 558 lesions were treated in 402 consecutive patients. Clinical follow-up was available at 15.7±10.2 months for 365 (91%) patients. Of 402 patients, 135 (33.6%) had claudication, 37 (9.2%) had ischaemic rest pain and 230 (57%) exhibited ischaemic ulcerations. Lesions were mostly identified in the femoropopliteal segments (55%), followed by below-the-knee (BTK) segments (32%). Complex TASC C/D lesions and moderate to severe calcifications (PACSS score ≥2) were present in 331 (82%) and 323 (80%) patients, respectively. The mean lesion length was 20.6±14.3 cm. Five (1%) perforations and 10 (2%) asymptomatic embolisations occurred. Bail-out stenting was performed in 4%, 16% and 3% of patients with common femoral artery, femoropopliteal and BTK lesions, respectively. During follow-up, 5 (3.9%) patients with claudication and 52 (21.9%) patients with critical limb-threatening ischaemia (CLTI) died (hazard ratio [HR] 3.7; p<0.001). Freedom from TLR was 87.5% (112 of 128) in patients with claudication and 82.3% (195 of 237) in patients with CLTI, respectively (HR 1.8; p=0.03).
Conclusions: The Phoenix atherectomy can be safely performed in patients with complex lesions with a relatively low rate of bail-out stenting and clinically acceptable TLR rates. German Clinical Trials Register: DRKS00016708
Introduction
Peripheral artery disease (PAD) is an endemic disease which currently affects more than 200 million individuals worldwide1. With an ageing population, this number is expected to further rise in future decades. Despite progress with pharmacologic treatments, these patients face high morbidity and mortality rates2.
Endovascular treatment for PAD is continuously evolving with proven clinical benefits such as reduced limb pain in patients with claudication and limb salvage in patients with critical limb-threatening ischaemia (CLTI)34. However, standard endovascular therapies such as balloon angioplasty and stent implantation have a limited effect in severely calcified lesions. In particular, lesions with circumferential calcifications result in inadequate balloon and stent expansion and consequently have poor long-term patency56. We, and others, have previously reported on the safety and short-term efficacy of the Phoenix (Philips) atherectomy device for the treatment of peripheral lesions789. Although these findings were promising, long-term results are still lacking.
Methods
Study design
We performed a single-centre prospective study, aiming to assess the demographic, clinical, procedural and follow-up data in patients treated using the Phoenix atherectomy device. Out of 1,335 patients referred to our department between July 2017 and May 2021 for endovascular PAD treatment, 402 consecutive patients underwent a Phoenix atherectomy and were prospectively included in our registry. Our trial was registered on the German Clinical Trials Register website (DRKS00016708). Approval was obtained from our local ethics committee (S-100/2017) of the University of Heidelberg and all patients provided written informed consent.
Typically, complex Transatlantic Inter-Society Consensus (TASC) C and D, strongly calcified lesions, especially in moving, no-stent zones like the common femoral (CFA) and the popliteal artery, were considered for atherectomy based on our internal standard operating procedures7910.
Patient population
Demographic data, cardiovascular risk factors and laboratory markers (haemoglobin, estimated glomerular filtration rate [GFR], Hba1c and high-sensitive troponin T [hsTnT]) were analysed.
Endovascular procedures
All procedures were performed by two experienced interventional angiologists (S. Giusca and G. Korosoglou), board certified for the endovascular treatment of PAD by the German Societies of Angiology and Cardiology. Details regarding our endovascular protocols were reported previously711. In short, lesions were passed with an antegrade or, if failed, with a retrograde approach11. Generally, care was taken to maintain the wire in the intraluminal space throughout the lesion. In this regard, the guidewires with high penetrating power, loaded on straight and angled support catheters, were used if necessary to facilitate intraluminal passage at the discretion of the operator. In case of a failed antegrade passage, because the wire entered or remained in the subintimal space, the retrograde approach was chosen after puncture of the pedal and crural vessels or the distal superficial femoral artery (SFA) to maintain intraluminal passage 11. If intraluminal passage could not be obtained from either antegrade or retrograde passage, atherectomy was deferred in accordance with the instructions for use (IFU), which warn against usage of the device in the subintimal space.
The Phoenix atherectomy device was used prior to balloon angioplasty. It consists of a catheter which houses the cutter at its distal tip. The cutter rotates at high speed, between 10,000 and 12,000 rpm, so that fragmented debris is removed due to strong suction forces. This obviates the need for filter protection. The Phoenix system is delivered over a 0.014-inch guidewire and can be used for the treatment of vessels with a diameter between 2.4 mm and 7 mm. Thus, depending on the vessel diameter, 1.5 mm, 1.8 mm, 2.2 mm and 2.4 mm Phoenix devices were used. The 1.5 mm (4 Fr) device was used for the treatment of vessels with diameters of 2.0-3.0 mm (typically in below-the-knee [BTK] crural and pedal arteries), the 1.8 mm (5 Fr) device for vessels with diameters of 2.5-4.5 mm (typically in BTK crural arteries), the 2.2 mm (6 Fr) device for vessels with diameters of 3.0-6.0 mm (typically in proximal crural, popliteal and SFA), and the 2.4 mm (7 Fr) deflecting device for vessels with diameters >4.5 mm (typically in popliteal, SFA and CFA). Following atherectomy, lesions were treated with plain old balloon angioplasty (POBA) followed by drug-coated balloons (DCB). In case of persistent recoil or flow-limiting dissections, self-expanding bare metal stents were implanted. Patients were treated medically with a combination of aspirin (100 mg daily) and clopidogrel for 1-3 months depending on their ischaemic and bleeding risk. Alternately, the VOYGER PAD regime was used12.
In all patients, duplex sonography was performed the day after the procedure to investigate vessel patency and to detect potential complications such as arteriovenous fistula and pseudoaneurysms at the proximal or distal access sites. Furthermore, an ankle-brachial index (ABI) measurement was performed the day before and the day after the procedure.
Clinical follow-up and study endpoints
Follow-up was conducted primarily through a structured clinical interview over the telephone with the patients or their families. The questions asked aimed at identifying the clinical status of the treated limb (i.e., improvement of walking distance for patients with claudication and/or healing of the wound in patients with CLTI), occurrence of minor or major (above the ankle) limb amputations and other cardiovascular events, such as myocardial infarction and stroke. In the case of patient death, the possible cause of death was interrogated. If either the patients or their respective families were not available, their referring clinician was contacted. Lastly, for all patients, a thorough review of the medical records was performed to identify repeat peripheral or coronary revascularisations, cardiovascular events, and possible causes of death. The follow-up was performed by a trained research assistant (S. Hagstotz) and was supervised by an experienced attending physician (S. Giusca). Clinical follow-up was performed at least 3 months after the index procedure.
Analysis of angiographic data
The TASC classification was used for determining the lesion complexity, localisation, and calcification on angiograms13. The degree of lesion calcification was categorised by the Peripheral Arterial Calcium Scoring System (PACSS): grade 0 = no visible calcium; grades 1 and 2 = unilateral calcification <5 or ≥5 cm, respectively; and grades 3 and 4 = bilateral calcification <5 or ≥5 cm, respectively6910. In addition, the in-stent restenosis (ISR) classification proposed by Tosaka et al14 was used to differentiate between class I, focal ISR lesions <5 cm in length, class II, diffuse ISR lesions >5cm in length, and class III, total ISR occlusions.
Study endpoints
Safety endpoints: Occurrence of perforation or embolisation during the atherectomy procedure.
Procedural endpoints:
(i) Technical success, defined as residual stenosis <50% after atherectomy but prior to adjunctive treatment by angiographic criteria.
(ii) Procedural success, defined as residual stenosis <30% after atherectomy and adjunctive treatment by angiographic criteria.
Long-term clinical endpoints: Freedom from clinically driven target lesion revascularisation (TLR) and improvement of at least one Rutherford (RF) category during follow-up.
Statistical analysis
Analysis was performed using the commercially available software MedCalc, version 18.5 (MedCalc). Continuous variables were expressed as mean±standard deviation and categorical variables as proportions. ABI measurements at baseline and after treatment were compared using a paired t-test. Categorical data were compared using chi-square tests. Kaplan-Meier analysis was used for the calculation of all-cause mortality and TLR rates during follow-up. In addition, Cox proportional hazards regression and multivariable regression models were assessed. The analysis of variance (ANOVA) test was used for comparing 3 or more normally distributed groups with the Scheffé test for post hoc analysis15. Differences were considered statistically significant at p<0.05.
Results
Demographic characteristics
Table 1 summarises demographic, laboratory, and procedural data. Patients were 76.5±9.9 years old, and 38% were female. Half of the patients (51%) had diabetes, and 181 (44%) had a history of coronary artery disease (CAD). Most patients, 375 (93%), were on lipid-lowering therapy. Patients with CLTI were older and exhibited more comorbidities, including renal failure, lower haemoglobin and elevated hsTnT values.
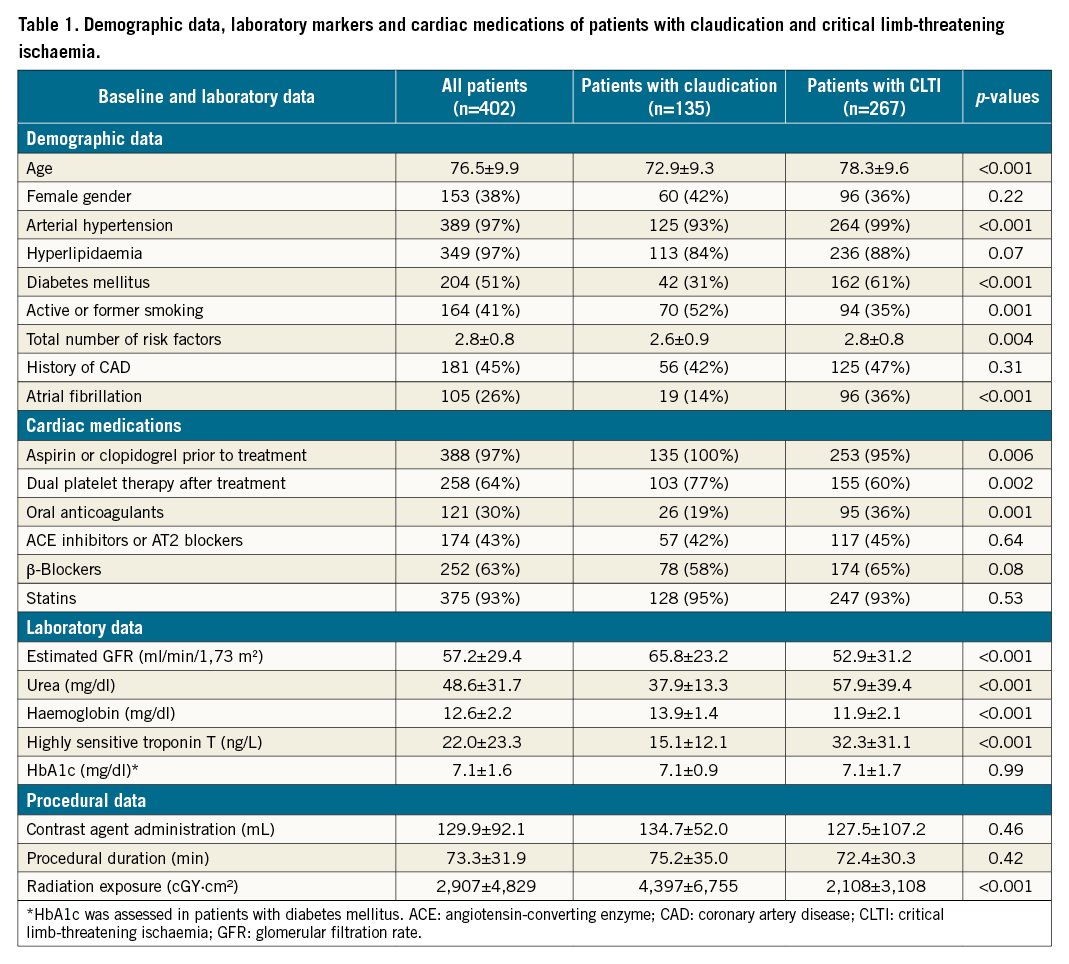
Clinical presentation
Of 402 patients, one third (33.6%) had intermittent claudication (RF category 2 or 3) and 9.2% had ischaemic rest pain (RF category 4). Over half of our cohort (57%) had ischaemic ulceration (RF category 5). The mean RF category was 4.3±1.0.
Lesion localisation and characteristics
Complex TASC C or D lesions were present in 331 (82%) patients, whereas lesions with at least moderate or severe calcifications (PACSS score ≥2) were present in 323 (80%) patients. Chronic total occlusion (CTO) lesions were present in 234 (58%) of the patients (1.1±0.3 CTO lesions per patient). The mean lesion length was 20.6±14.3 cm. Of the 31 (8%) patients with ISR lesions, 9 had class I, 3 had class II, and 19 had class III CTO lesions. Retrograde recanalisation was performed in 57 (14%) patients. Most lesions were in the femoropopliteal (55%), followed by the BTK (32%), segments. Patients with CLTI more commonly had lesions BTK than in femoropopliteal segments, they also had longer lesion lengths and more CTO lesions (Table 2).
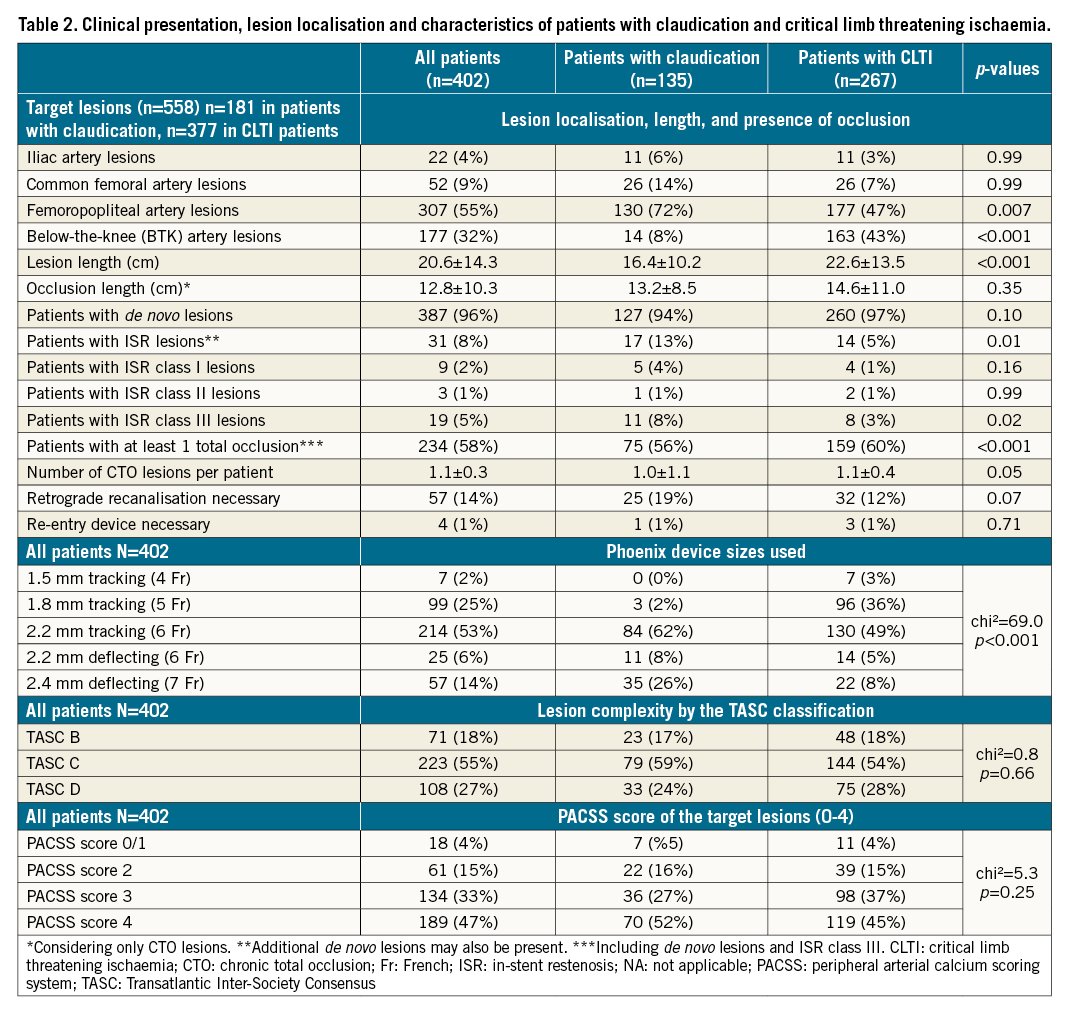
Correlative analysis
Lesion length increased with the TASC classification and PACSS score (Supplementary Figure 1A, Supplementary Figure 1B). In addition, an elevated PACSS score was associated with higher lesion complexity by TASC as well as with higher hsTnT values (Supplementary Figure 1C, Supplementary Figure 1D).
Technical and procedural success and complication rates
The 1.5 mm, 1.8 mm and 2.2 mm tracking and the 2.2 mm and 2.4 deflecting devices were used in 2%, 25%, 53%, 6% and 14% of our patients, respectively (Table 2). The 2.4 mm and 2.2 mm deflecting tracking devices were mostly used in iliac and CFA lesions (61%; 45 of 74); 2.2 mm tracking devices in femoropopliteal (66%; 203 of 307) and 1.8 mm tracking devices in BTK (54%; 96 of 177) lesions (Supplementary Figure 2).
Technical and procedural success rates were 60% (243) and >99% (400), respectively. Perforations occurred in 5 (1%) patients; 3 of the cases were managed by prolonged balloon inflation and 2 cases required the implantation of a covered stent. Peripheral embolisation occurred in 10 (2%) patients, which were all managed by using catheter aspiration, without persistent vessel occlusions, clinical symptoms, or the need for local lysis (Table 3). Notably, no perforation, embolisation or device entrapment occurred during atherectomy in ISR lesions.
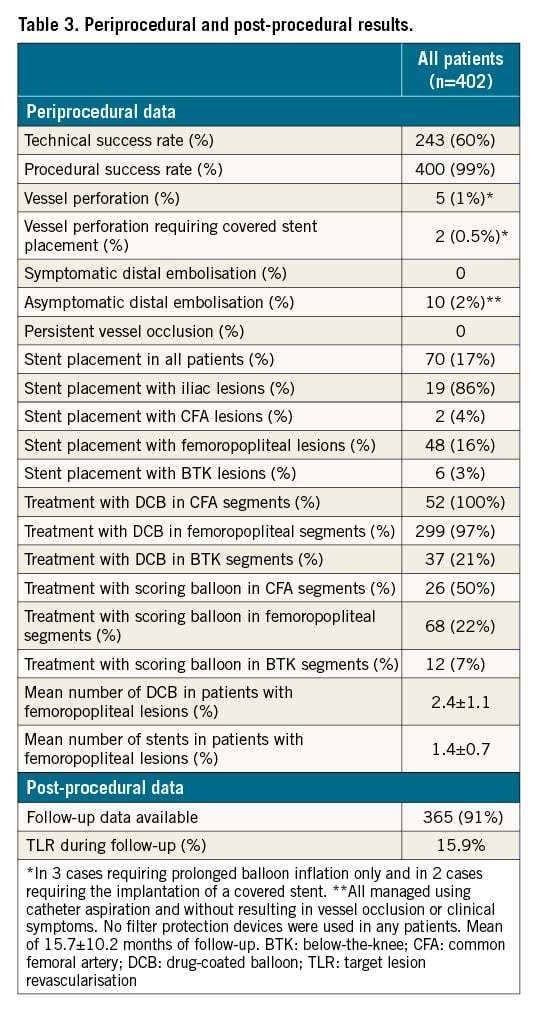
Adjunctive treatment
For iliac, CFA, femoropopliteal and BTK lesions, DCB were used in 14%, 100%, 97% and 21%, respectively. The mean number of DCB for femoropopliteal lesions, was 2.4±1.1 per patient. Stent implantation was necessary in 86% of patients with iliac lesions and in 2%, 16% and 3% of patients with CFA, femoropopliteal and BTK lesions, respectively (Table 3).
ABI and follow-up data
ABI of the affected limbs improved from 0.4±0.3 to 0.9±0.1 after treatment (p<0.001). Clinical follow-up was available at 15.7±10.2 months for 365 (91%) patients. During follow-up, 5 (3.9%) patients with claudication and 52 (21.9%) patients with CLTI died (HR 3.7; p<0.001) (Central illustration A). Death was attributed to cardiovascular causes in 24 patients, sepsis and multiorgan failure in 22 patients, and sudden death or death of unknown cause in 11 patients. No procedural-related deaths occurred.
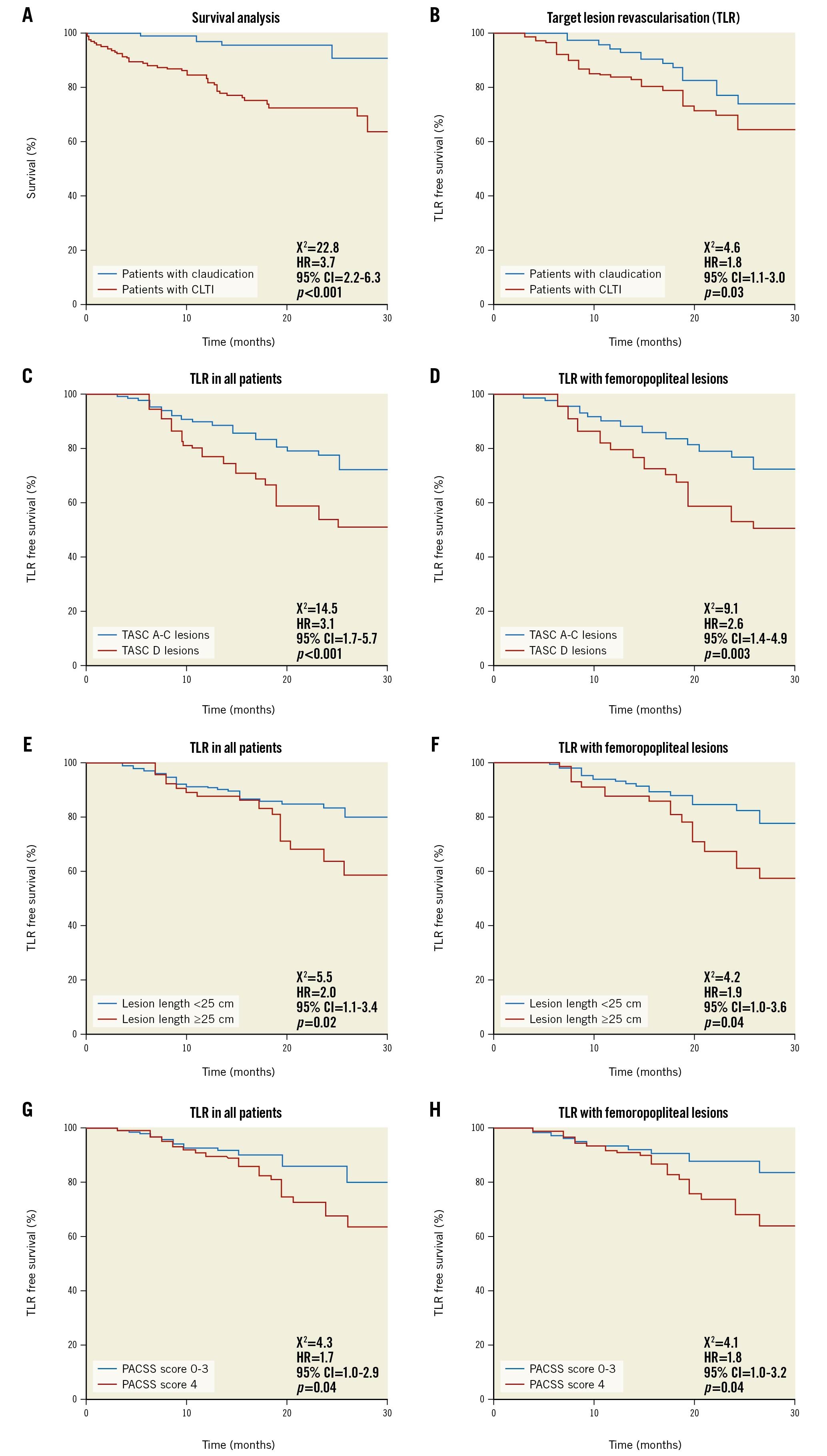
Central illustration. All-cause mortality and freedom from TLR in patients with claudication versus CLTI. A) All-cause mortality was significantly higher in patients with claudication compared to CLTI. B) Freedom from TLR during follow-up was higher in patients with claudication compared to patients with CLTI. C-D) Freedom from TLR was lower in patients with TASC D versus TASC A-C lesions, (E-F) in patients with ≥25 cm versus <25 cm lesions and (G-H) in patients with severe calcifications PACSS score 4 versus 0-3. This was the case in all patients and in patients with femoropopliteal lesions (C-H). CI: confidence interval; CLTI: critical limb-threatening ischaemia; HR: hazard ratio; PACSS: peripheral arterial calcium scoring system; TASC: Transatlantic Inter-Society Consensus; TLR: target lesion revascularisation
Minor and major amputations were performed in 41 (15.4%) and 3 (1.1%) patients with CLTI, respectively; whereas there was only 1 (0.7%) minor amputation in a patient with claudication who progressed to CLTI, due to a reoccluded SFA 16 months after the index procedure.
Freedom from TLR was 84.1% at 15.7 months of follow-up in all patients, 87.5% (112 of 128) in patients with claudication and 82.3% (195 of 237) in patients with CLTI, respectively (HR 1.8; p=0.03) (Central illustration B). In all patients and in patients with femoropopliteal lesions, TLR rates were higher in patients with TASC D versus TASC A-C lesions, patients with ≥25 cm versus <25 cm lesions, and in patients with PACSS score 4 versus 0-3, respectively (Central illustration C-Central illustration H). A subsection analysis for >10 cm, 10-25 cm and >25cm lesions is also provided (Supplementary Figure 3). The estimated TLR rates are shown in Supplementary Table 1.
Using the Cox proportional hazards regression models, hsTnT and CLTI were predictive for all-cause death, whereas lesion length was the strongest predictor for TLR (Table 4A, Table 4B). In addition, lesion length, TASC classification and the presence of CTO, but not calcification by PACSS, were all independent predictors of bail-out stenting with femoropopliteal lesions (Table 4C).
Clinical improvement by at least one Rutherford category was observed in 95.3% of patients with claudication, 87.5% of patients with CLTI and 90.2% of all patients (Figure 1).
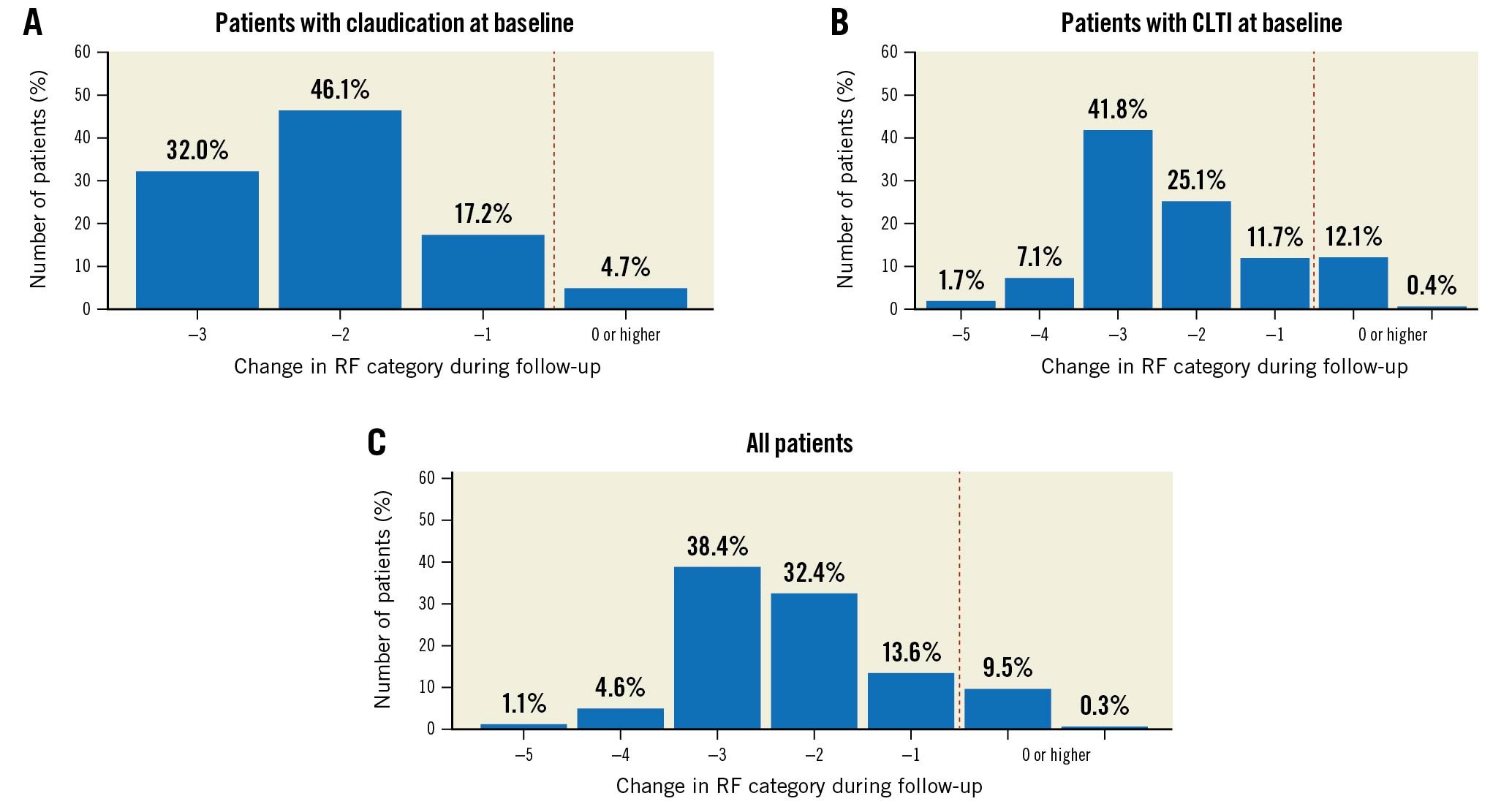
Figure 1. Changes of RF categories (A) in patients with claudication, (B) in CLTI patients and (C) in all patients during follow-up. CLTI: critical limb-threatening ischaemia; RF: Rutherford
Representative cases
Two examples of patients who underwent a Phoenix atherectomy, (i) with complex and calcified lesions of the CFA and SFA (RF category 3), and (ii) with BTK lesions (RF category 5) are provided in Figure 2 and Supplementary Figure 4, respectively. In addition, 3 patients who underwent a Phoenix atherectomy for a focal and a diffuse ISR lesion, as well as for an ISR CTO, are shown in the Supplementary Figure 5.
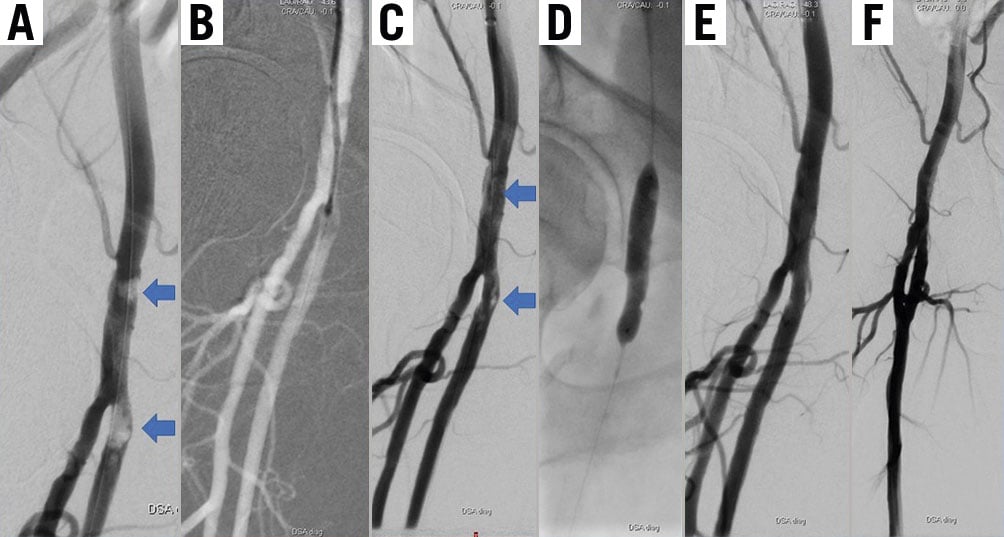
Figure 2. Patient presenting with RF category 3 due to calcified lesions in the common femoral and at the origin of the superficial femoral artery. A) After several passages with the 2.4 mm deflecting atherectomy device B) good lumen gain can be observed (blue arrows in C), which is further optimised using scoring balloon (D) and DCB angioplasty with a good final result (E-F). The patient remained uneventful at 13 months of follow-up. DCB: drug-coated balloons; RF: Rutherford
Discussion
In this prospective, all-comers observational study, we systematically analysed patients and lesions characteristics in consecutive patients with symptomatic PAD who underwent atherectomy for complex and calcified peripheral lesions. We found that the Phoenix atherectomy device is safe and effective for the endovascular treatment of such lesions, demonstrating low rates of perforation and peripheral embolisation. In addition, TLR rates were clinically acceptable at 12.5% and 17.7% in patients with claudication and CLTI, respectively. TLR rates were higher in patients with TASC D, PACSS 4 lesions, and in lesions >25cm.
PAD is associated with increased morbidity and mortality as well as increased costs for health systems4. Patients often suffer from increased disability and reduced quality of life1617. In this regard, endovascular treatment of PAD is widely recognised as the first treatment of choice, especially in elderly and frail patients18. Recently, it was shown that an increase in the number of endovascular procedures drives rising costs in health care systems. However, this was associated with markedly decreasing rates of major amputations, which is a crucial clinical goal4. Endovascular procedures are usually performed in specialised centres since different approaches and devices need to be employed according to the localisation and complexity of the underlying lesions19. In particular with femoropopliteal lesions, DCB angioplasty was shown to increase long-term patency even in complex lesions, obviating the need for stent placement20. However, with severe circumferential calcification, the amount of the drug absorbed from the DCB is significantly reduced, which diminishes local pharmacologic effectiveness for the prevention of restenosis6. The combination of DCB and stent placement, on the other hand, may also prove inefficient in strongly calcified lesions, leading to ISR, stent fracture and higher reocclusion rates with such “leave behind” strategies2122.
Since balloon angioplasty and stent placement only press atherosclerotic material to the vessel wall, it is conceivable that complex lesions, consisting of calcified and fibrous tissue, would benefit from debulking strategies. In this regard, atherectomy has been proposed, providing luminal gain and thus a good “working channel”, facilitating more homogeneous balloon expansion during subsequent angioplasty at low inflation pressures and simultaneously higher deliverability of drugs into the vessel wall with the DCB1023 .
Previously, we reported high procedural success and low complication rates in 136 patients who underwent a Phoenix atherectomy7. Our current data in 402 patients confirm these previously reported high procedural success rates of <99% with low complication rates. Notably, in our registry, the Phoenix device was used only after intraluminal wire passage since its use in the subintimal space is not recommended by the IFU; this is also the case with most currently available devices. In addition, no complications occurred during the treatment of ISR lesions and CTO in 31 patients, although the Phoenix device is not specifically approved for this anatomical region. However, since the risk of perforation within implanted stents is reasonably low, in our experience atherectomy can be performed safely as demonstrated in Supplementary Figure 5. Since our study did not focus on ISR lesions, this issue merits further investigation in future studies.
An overview of different atherectomy devices and corresponding restrictions by IFU for their use in the subintimal space and with ISR lesions is provided in Supplementary Table 2.
The rate of peripheral embolisation was relatively low despite the lack of filter protection in all patients. It is difficult to predict this potentially devastating complication through clinical history and angiographic images. The role of intravascular ultrasound (IVUS) in the detection of pre-existing thrombi prior to treatment may be decisive to further reduce the rates of this complication in future studies. In addition, catheter aspiration proved effective for the removal of embolised debris. This manoeuvre may be easy to perform when an antegrade puncture of the ipsilateral side is selected but is cumbersome due to limited catheter lengths when using a crossover approach.
Follow-up data demonstrated good freedom from clinically driven TLR in 84.1% of patients at 15.7 months after the index procedure, translating in an estimated freedom from TLR of 89.5% at 1 year and 75.0% at 2 years of follow-up in all patients (Supplementary Table 1). Patients with claudication had lower rates of reintervention compared with patients who were treated for CTLI, which is in agreement with previous reports24. In addition, sustained improvement of an RF category was observed in over 90% of all patients and in over 95% of patients with claudication. Particularly in such patients, sustained clinical improvement during follow-up is an important clinical goal and implicates the durability of our treatment regime. To the best of our knowledge, this is the first study to evaluate the long-term effectiveness of a rotational atherectomy device in combination with conventional endovascular treatments (POBA, DCB and stents) in such a large patient cohort and to provide long-term follow-up data.
In our registry, bail-out stenting was low with CFA and BTK but higher with femoropopliteal lesions. Lesion length was an independent predictor of clinically driven TLR, whereas lesion length, complexity by TASC classification as well as the presence of CTO were independent predictors for bail-out stenting with femoropopliteal lesions. Interestingly, lesion calcification was not independently associated with TLR or bail-out stenting, which may be due to the limitation of fluoroscopy to address the presence of circumferential calcifications of the vessel wall.
Directional, rotational, and orbital atherectomy devices are currently available1023. The Jetstream rotational atherectomy device (Boston Scientific) was previously evaluated in 172 patients with infra-inguinal lesions, demonstrating 1-year freedom from TLR of 74%25. Furthermore, the COMPLIANCE 360° trial evaluated orbital atherectomy in 50 patients and reported a patency rate of 80% at 1 year26. The DEFINITIVE AR study, on the other hand, tested a directional atherectomy device in 102 patients, demonstrating freedom from TLR of 92.7% at 1 year27. These TLR rates cannot be compared with our study, due to the variation of patients with different demographic and lesion characteristics within different trials. However, it may be noted that freedom from TLR was 89.5% and 70% at 1 year and 2 years, respectively, in our study, which is quite high considering the high rate of complex TASC C/D and calcified lesions. In addition, other studies with directional atherectomy devices found relatively high rates of acute complications such as embolisation (3.8%), dissection (2.3%) and perforation (5.3%), compared to our study28.
We found a relatively high rate of mortality in patients with CLTI of 16.5% at 1 year and 27.2% at 2 years. This corresponds to previous reports, which showed mortalities varying from 15% to 30% at 1 year and reaching 50% at 5 years in such patients29. This is mostly related to the increased number of comorbidities found in these patients. In addition, hsTnT, an established marker of cardiovascular risk, was markedly elevated in CLTI patients, underscoring increased cardiovascular risk. It should be noted, however, that the higher mortality in CLTI patients is difficult to support through registry data, as provided in our study, due to the presence of several confounders and comorbidities which were not controlled by the study protocol due to the lack of randomisation and patient matching.
Limitations
Several limitations of our study need to be acknowledged. Firstly, this was a prospective single-arm study. Thus, no comparison to other devices is provided. As well, no control arm consisting of conventional endovascular therapies without atherectomy is provided. A recent Cochrane meta-analysis detected a trend for lower dissection and stenting rates after minimally invasive atherectomy. However, the question of the benefit of atherectomy on limb and patient outcomes on top of conventional endovascular therapy could not be addressed30, which underlines the need for future randomised controlled trials. In addition, clinically driven TLR was selected as the primary endpoint, whereas data on vessel patency based on duplex ultrasound and serial ABI measures are not available. However, clinically driven TLR is an important clinical endpoint, since repeated interventions are associated with higher costs, low quality of life and increased risks, from both the physicians’ and patients’ perspectives. Finally, atherectomy was performed only after antegrade or retrograde intraluminal lesion passage, based on IFU for the Phoenix device, which warns against the use of the device after subintimal wire passage. Thus, perforation rates may have been >1% in the case of atherectomy within the subintimal space. However, since IVUS was not routinely performed in most of our patients, at least partial subintimal lesion passage cannot be completely excluded with CTO lesions in our study.
Conclusions
A Phoenix atherectomy exhibits an excellent safety profile for the treatment of patients with complex, calcified peripheral lesions with a relatively low rate of bail-out stenting in infra-inguinal vessel segments and clinically acceptable TLR rates. Future trials are now warranted, addressing the incremental value of this technique compared with standard treatment in terms of safety, effectiveness, and outcomes.
Impact on daily practice
In our prospective, all-comers, single-centre trial, we found that atherectomy is safe and effective for the endovascular treatment of patients with moderately to severely calcified lesions, exhibiting high procedural success and very low complication rates. In addition, the clinical success rates were high with sustained clinical improvements during follow-up and a low rate of clinically driven TLR. Considering the relatively low stent implantation rate, these results appear very promising from an endovascular point of view and may help to change daily clinical practice towards an endovascular or “atherectomy first” strategy with moderately to severely calcified infra-inguinal peripheral lesions.
Funding
GRN Hospital Weinheim (G. Korosoglou & S. Giusca) received an institutional research grant from Volcano and Philips.
Conflict of interest statement
G. Korosoglou received speaker honoraria from Volcano, Philips and meeting/travel support from Philips. M. Lichtenberg received speaker honoraria from Volcano and Philips, payment for expert testimony from Philips, meeting/travel support from Philips and is past president of the German Angiology Society. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

