Abstract
Background: There is a paucity of real-world data on the in-hospital (IH) and post-discharge outcomes in patients undergoing lower extremity peripheral vascular intervention (PVI) with adjunctive atherectomy.
Aims: In this retrospective, registry-based study, we evaluated IH and post-discharge outcomes among patients undergoing PVI, treated with or without atherectomy, in the National Cardiovascular Data Registry PVI Registry.
Methods: The IH composite endpoint included procedural complications, bleeding or thrombosis. The primary out-of-hospital endpoint was major amputation at 1 year. Secondary endpoints included repeat endovascular or surgical revascularisation and death. Multivariable regression was used to identify predictors of atherectomy use and its association with clinical endpoints.
Results: A total of 30,847 patients underwent PVI from 2014 to 2019, including 10,971 (35.6%) treated with atherectomy. The unadjusted rate of the IH endpoint occurred in 524 (4.8%) of the procedures involving atherectomy and 1,041 (5.3%) of non-atherectomy procedures (p=0.07). After adjustment, the use of atherectomy was not associated with an increased risk of the combined IH endpoint (p=0.68). In the 6,889 (22.4%) patients with out-of-hospital data, atherectomy was associated with a reduced risk of amputation (adjusted hazard ratio [aHR] 0.67, 95% confidence interval [CI]: 0.51-0.85; p<0.01) and surgical revascularisation (aHR 0.63, 95% CI: 0.44-0.89; p=0.017), no difference in death rates (p=0.10), but an increased risk of endovascular revascularisation (aHR 1.21, 95% CI: 1.06-1.39; p<0.01) at 1 year.
Conclusions: The use of atherectomy during PVI is common and is not associated with an increase in IH adverse events. Longitudinally, patients treated with atherectomy undergo repeat endovascular reintervention more frequently but experience a reduced risk of amputation and surgical revascularisation.
Introduction
Patients with symptomatic peripheral artery disease (PAD) experience significant rates of restenosis following endovascular revascularisation1. Vessel preparation involves increasing luminal gain and modifying plaque before standard percutaneous transluminal angioplasty is performed, with the intention of improving technical success and improving long-term patency following peripheral vascular intervention (PVI). Various atherectomy devices are utilised for atherosclerotic vessel preparation, with the goal of relieving lesion-related stenosis, reducing the need for high-pressure angioplasty, thus reducing associated dissection and perforation risks, and increasing arterial permeability by fracturing plaque and removing calcium to optimise drug delivery to the vessel wall23456. Although a range of vessel preparatory devices are available, a contemporary understanding of device utilisation, practice patterns and outcomes has not been well characterised.
Prospective non-randomised data suggest the use of vessel preparation techniques improves outcomes following PVI78. However, the limited data from randomised trials comparing drug-coated balloon (DCB) alone versus DCB in combination with atherectomy in complex lesions indicate a high level of technical success with a combined approach but no improvement in longitudinal clinical outcomes910. Furthermore, as a result of the limited available prospective data, concerns remain about the procedural risks of embolism and dissection with atherectomy911. Despite the potential for improved outcomes as well as concerns of procedural risk, there are sparse data on the practice patterns and short- and long-term outcomes among patients who undergo vessel preparation at the time of PVI121314.
The aim of this study was to describe physician practice patterns in the use of vessel preparation techniques during PVI and to examine predictors of clinical outcomes during index revascularisation for lower extremity PAD. To accomplish this, the National Cardiovascular Data Registry (NCDR) PVI Registry linked with longitudinal Medicare data was used to characterise nationwide physician practice patterns, predictors of need for vessel preparation, and outcomes among patients undergoing infrainguinal PVI.
Methods
STUDY POPULATION
The NCDR PVI Registry is a prospective, independent collection of patient, hospital, and procedural characteristics and outcomes from individual patients from over 200 centres in the USA15. Collected data are transmitted to the NCDR through certified processes and then undergo quality assurance review and auditing; data from hospital-based procedures from the various participating centres operating as outpatient-based laboratories (OBLs) are not included in the NCDR. A waiver of written informed consent and authorisation for this study was granted by the Partners Health Institutional Review Board. All patients (≥66 years) were linked to the Medicare claims database. For Medicare-linked data, patients were assessed from the 2nd quarter of 2014 to the 4th quarter of 2017.
INCLUSION AND EXCLUSION CRITERIA
Consecutive patients undergoing PVI were eligible for inclusion. Patients with no data on the treated lesion segment, patients with graft lesions, and patients with acute limb ischaemia (ALI) were excluded (Figure 1). We identified all patients from the NCDR PVI Registry who underwent PVI with or without atherectomy − defined as the use of rotational, directional, or orbital atherectomy as documented in the Registry’s case report form during the index PVI − from the 2nd quarter of 2014 to the 2nd quarter of 2019. Only patients receiving femoral, popliteal, or tibial atherectomy were included. Patients undergoing iliac PVI and those treated with laser atherectomy were excluded.
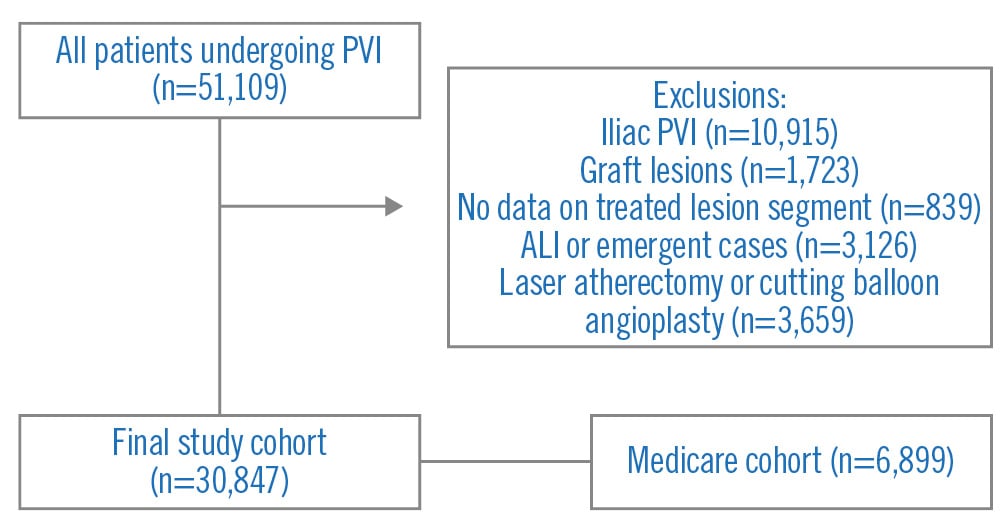
Figure 1. Study design. ALI: acute limb ischaemia; PVI: peripheral vascular intervention
EXPOSURES
The primary exposures were atherectomy with or without stenting compared to plain old balloon angioplasty with or without stenting (POBA±stent).
OUTCOMES
The primary in-hospital (IH) safety composite endpoint was defined as procedural dissection, perforation, embolism, thrombosis, compartment syndrome, major amputation, emergent vascular surgery, unplanned vascular intervention(s), or major bleeding. The primary out-of-hospital safety endpoint was major amputation at 1 year. Secondary out-of-hospital endpoints included repeat endovascular revascularisation of either limb, surgical revascularisation and death. International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Diseases, Tenth Revision, Clinical Modification claims codes were used to define the out-of-hospital endpoints of major amputation, repeat endovascular or surgical revascularisation, and death up to 1 year (Supplementary Table 1)16.
STATISTICAL METHODS
All metrics and normally distributed variables are reported as mean±standard deviation and were compared using the Student’s t-test. Categorical variables are presented as frequency and percentage and were compared with the chi-square test.
Using multivariable logistic regression analyses, we also examined predictors of atherectomy use during PVI, including the anatomical location and severity of the target lesion, procedural indication, procedure location (endovascular or operative suite), procedural status (urgent or elective), and procedural technique. Predictive variables were based on clinical judgment of factors believed or known to be associated with atherectomy use.
Next, we defined our primary in-hospital outcome as a composite of dissection, perforation, embolism, thrombosis, compartment syndrome, major amputation, emergent vascular surgery, unplanned vascular intervention, or major bleeding. We then used a multivariable logistic regression model to derive the adjusted odds ratios (OR) of atherectomy on our primary outcome after adjusting for segment location, indication, multiple lesions, procedure location, non-elective status, subintimal strategy, age, gender, insurance, diabetes mellitus, dialysis, left ventricular systolic dysfunction, race, lung disease, coronary artery disease, chronic total occlusion lesion, prior cardiovascular disease, and drug-coated balloon use.
Finally, we used procedures from 2014-2017 that were linked to Medicare to determine the long-term outcomes of mortality, endovascular revascularisation, surgical revascularisation, and amputation (Table 1, Supplementary Table 1). We then used Kaplan-Meier analysis to determine rates at 1 year and used multivariable proportional hazards models to determine the adjusted hazard ratio (HR) of atherectomy on outcomes. All analyses were performed with SAS version 9.4 (SAS Institute).
Table 1. Demographic and procedural characteristics.
| Total (N=30,847) |
Atherectomy (N=10,971) |
POBA±stent (N=19,876) |
p-value | ||
|---|---|---|---|---|---|
| Age, yrs | 69.8±11.3 | 70.4±10.8 | 69.4±11.5 | <0.001 | |
| Female | 12,345 (40.0) | 4,313 (39.3) | 8,032 (40.4) | 0.06 | |
| African American | 5,648 (18.3) | 1,800 (16.4) | 3,848 (19.4) | <0.001 | |
| Hispanic or Latino | 2,103 (6.8) | 825 (7.5) | 1,278 (6.5) | <0.001 | |
| Coronary artery disease | 16,069 (52.1) | 5,959 (54.4) | 10,110 (50.9) | <0.001 | |
| Myocardial infarction | 47 (0.2) | 16 (0.1) | 31 (0.2) | 0.824 | |
| Hypertension | 28,080 (91.1) | 10,104 (92.2) | 17,976 (90.5) | <0.001 | |
| Dyslipidaemia | 25,237 (81.9) | 9,195 (83.9) | 16,042 (80.8) | <0.001 | |
| Diabetes mellitus | 18,502 (60.0) | 6,688 (61.0) | 11,814 (59.5) | <0.01 | |
| Prior heart failure | 6,208 (20.1) | 2,157 (19.7) | 4,051 (20.4) | 0.14 | |
| Severe lung disease | 4,391 (14.3) | 1,533 (14.0) | 2,858 (14.4) | 0.34 | |
| Tobacco use − current | 9,600 (31.2) | 3,200 (29.2) | 6,400 (32.2) | <0.001 | |
| ESRD on dialysis | 2,854 (9.3) | 1,130 (10.3) | 1,724 (8.7) | <0.001 | |
| Ankle-brachial index performed | 14,135 (45.9) | 4,592 (41.9) | 9,543 (48.1) | <0.001 | |
| Ankle-brachial index value | 0.8±0.3 | 0.8±0.3 | 0.8±0.3 | 0.20 | |
| PVI indication | Typical claudication | 13,357 (43.3) | 5,301 (48.4) | 8,056 (40.6) | <0.001 |
| Atypical claudication | 755 (2.5) | 221 (2.0) | 534 (2.7) | ||
| Maintenance of patency | 280 (0.9) | 55 (0.5) | 225 (1.1) | ||
| Critical limb ischaemia | 16,194 (52.6) | 5,343 (48.8) | 10,851 (54.6) | ||
| Prevention of aneurysm | 35 (0.1) | 1 (0.0) | 34 (0.2) | ||
| Aneurysm | 77 (0.2) | 2 (0.0) | 75 (0.4) | ||
| Facilitation of other procedure | 116 (0.4) | 29 (0.3) | 87 (0.4) | ||
| Procedure location | Catheterisation lab | 25,831 (83.8) | 9,664 (88.2) | 16,167 (81.4) | <0.001 |
| Interventional radiology | 3,501 (11.4) | 945 (8.6) | 2,556 (12.9) | ||
| Operating room | 1,500 (4.9) | 354 (3.2) | 1,146 (5.8) | ||
| Insurance payers | Private health insurance | 18,419 (60.6) | 6,589 (60.6) | 11,830 (60.6) | 0.95 |
| Medicaid | 5,452 (17.9) | 1,884 (17.3) | 3,568 (18.3) | 0.04 | |
| Medicare | 21,683 (71.3) | 7,779 (71.5) | 13,904 (71.2) | 0.51 | |
| Military | 944 (3.1) | 360 (3.3) | 584 (3.0) | 0.12 | |
| State-specific | 674 (2.2) | 204 (1.9) | 470 (2.4) | <0.01 | |
| Anatomical location | Femoropopliteal | 25,087 (81.3) | 9,114 (83.1) | 15,973 (80.4) | <0.001 |
| Tibiopedal | 5,760 (18.7) | 1,857 (16.9) | 3,903 (19.6) | ||
| LESION CHARACTERISTICS | |||||
| Chronic total occlusion | 10,674 (34.6) | 3,666 (33.4) | 7,008 (35.3) | 0.001 | |
| Max lesion length, mm | 120.7±93.4 | 126.9±91.9 | 117.1±94.0 | <0.001 | |
| Thrombus present | 2,229 (7.2) | 507 (4.6) | 1,722 (8.7) | <0.001 | |
| Bifurcation lesion | 1,329 (4.3) | 595 (5.4) | 734 (3.7) | <0.001 | |
| Max stenosis prior to treatment, % | 92.6±14.3 | 93.8±9.2 | 91.9±16.5 | <0.001 | |
| Max stenosis post-treatment, % | 17.5±27.8 | 13.7±20.8 | 19.6±30.9 | <0.001 | |
| PROCEDURAL CHARACTERISTICS | |||||
| Atherectomy type | Rotational | 106 (1.0) | |||
| Orbital | 5,381 (49.0) | ||||
| Directional | 5,059 (46.1) | ||||
| More than 1 device | 99 (0.9) | ||||
| None listed | 326 (3.0) | ||||
| Angiographic run-off | 3V | 6,454 (28.3) | 2,476 (28.3) | 3,978 (28.3) | <0.001 |
| 2V | 7,276 (31.9) | 2,955 (33.7) | 4,321 (30.7) | ||
| 1V | 7,192 (31.5) | 2,743 (31.3) | 4,449 (31.6) | ||
| None | 1,913 (8.4) | 583 (6.7) | 1,330 (9.4) | ||
| Stent implanted | 9,676 (31.4) | 2,682 (24.4) | 6,994 (35.2) | <0.001 | |
| Covered stent | 1,439 (4.7) | 258 (2.4) | 1,181 (5.9) | <0.001 | |
| Drug-coated balloon | 11,661 (37.8) | 5,643 (51.4) | 6,018 (30.3) | <0.001 | |
| Intentional subintimal strategy | 1,121 (3.6) | 246 (2.2) | 875 (4.4) | <0.001 | |
| Contrast volume, ml | 123.2±74.7 | 128.5±73.3 | 120.3±75.3 | <0.001 | |
| Fluoroscopy time, min | 20.6±15.4 | 22.7±15.0 | 19.4±15.5 | <0.001 | |
| Data are mean±standard deviation or n (%). ESRD: end-stage renal disease; POBA: plain old balloon angioplasty; PVI: peripheral vascular intervention; V: vessel | |||||
Results
STUDY POPULATION
A total of 51,109 patients undergoing PVI from 1 April 2014 to 30 June 2019 were eligible. For the index PVI, typical claudication (43.3%, n=13,357) was the most common PVI indication (Table 1). The following were excluded: patients with no data on treated arterial segment (n=839), patients with graft lesions (n=1,723), patients undergoing iliac PVI (n=10,915), patients undergoing laser atherectomy or cutting balloon angioplasty (n=3,659) and those with ALI or emergent cases (n=3,126). The remaining 30,847 patients formed the study cohort (Figure 1), of whom 10,971 (35.6%) underwent atherectomy. Baseline and procedural variables are presented in Table 1.
The mean age of patients in both cohorts was 69.8±11.3 years, and 40.0% (n=12,345) of patients were female. Patients undergoing PVI treated with atherectomy were less commonly white and had a greater burden of cardiovascular conditions and risk factors, including coronary artery disease, end-stage renal disease, severe lung disease, congestive heart failure, diabetes, and hyperlipidaemia (Table 1). The most commonly used types of atherectomy were directional and orbital (Table 1). Patients treated with atherectomy were less likely to be current tobacco users. Critical limb ischaemia (CLI) was the indication for PVI in 52.6% (n=16,194) of all patients and in 48.8% (n=5,343) and 54.6% (n=10,851) of patients treated with atherectomy and POBA±stent, respectively (Table 1).
PVI SETTING AND PROCEDURAL CHARACTERISTICS
A cardiac catheterisation laboratory was the most common location for PVI in the entire study population (83.8%, n=25,831). Atherectomy (88.2%, n=9,664) was more commonly performed than POBA±stent (81.4%, n=16,167; p<0.001) at the time of PVI among patients treated in a cardiac catheterisation laboratory. POBA±stent was more commonly performed than atherectomy among patients treated in an interventional radiology suite or operating room; however, there were relatively few patients treated in these procedural locations in this study population (Table 1). Insurance coverage also differed among groups, with atherectomy-treated patients more likely to have Medicare but less likely to have Medicaid or state-specific insurance plans.
Overall, 31.4% (n=9,676) of patients received an arterial stent at the time of PVI including 24.4% (n=2,682) of patients in the atherectomy group and 35.2% (n=6,994) of patients in the POBA±stent group (p<0.001). The femoropopliteal arterial segment was the most commonly treated segment overall (81.3% of all PVIs). Notably, the femoropopliteal segment (83.1%, n=9,114) was the most common target lesion among patients treated with atherectomy and among patients treated with POBA±stent (80.4%, n=15,973) (Table 1). Covered stent use (2.4% vs 5.9%; p<0.001) was less common in the atherectomy group; however, DCB use was more common (51.4% vs 30.3%; p<0.001). The presence of thrombus, bifurcation lesions, or intentional subintimal revascularisation was uncommon in both groups. Lesions in the atherectomy-treated patients were more frequently of greater angiographic severity (93.8%±9.2% vs 91.9%±16.5%; p<0.001) and of greater length (126.9 mm±91.9 mm vs 117.1 mm±94.0 mm; p<0.001). Furthermore, the amount of contrast volume (128.5 ml±73.3 ml vs 120.3 ml±75.3 ml; p<0.001) and fluoroscopy time (22.7 min±15.0 min vs 19.4 min±15.5 min; p<0.001) were greater in patients treated with atherectomy.
PREDICTORS OF ATHERECTOMY USE
Independent predictors of atherectomy use are shown in Figure 2. Anatomical location and the extent of the target lesion were associated with atherectomy use. Femoropopliteal intervention was associated with a higher likelihood of atherectomy use compared to tibiopedal PVI (OR 1.12, 95% confidence interval [CI]: 1.05-1.20; p<0.001). In addition, multiple lesions were more likely to be treated with atherectomy compared to single lesions (OR 1.41, 95% CI: 1.34-1.49; p<0.001). Atherectomy use was less likely among patients presenting with critical limb ischaemia (OR 0.80, 95% CI: 0.76-0.85; p<0.001) compared to those with typical claudication. The procedural setting also influenced the odds of being treated with atherectomy – patients treated in an interventional radiology suite (OR 0.71, 95% CI: 0.65-0.77; p<0.001) or operating room (OR 0.62, 95% CI: 0.55-0.71; p<0.001) were less likely to receive atherectomy compared to those treated in a cath lab (Figure 2).
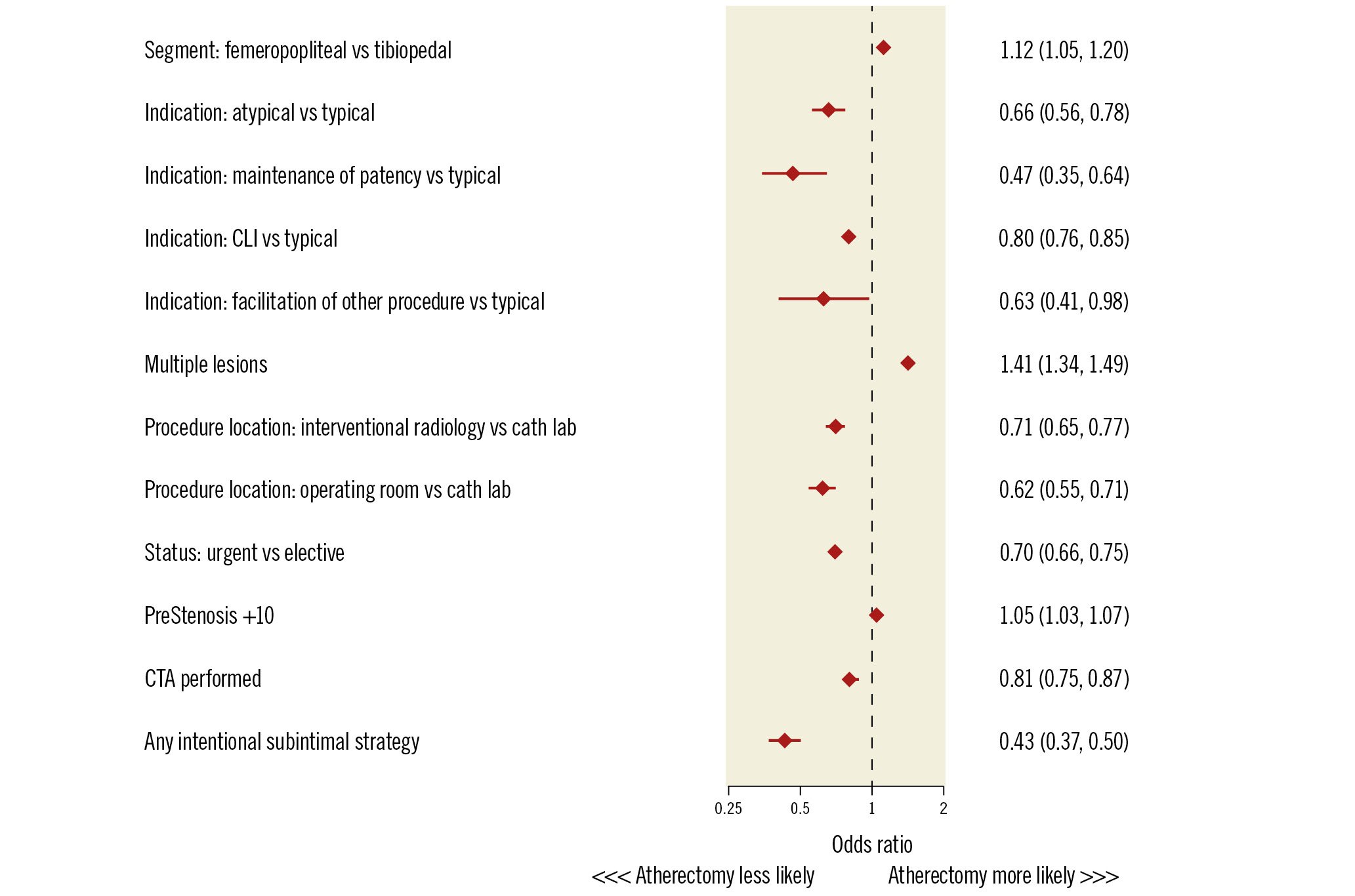
Figure 2. Forest plot comparing predictors of atherectomy. CLI: critical limb ischaemia; CTA: computed tomography angiography; PreStenosis+10: the additional risk accrued by every additional 10% increase in angiographic stenosis severity
IN-HOSPITAL OUTCOMES
Overall, the incidence of the in-hospital composite outcome of procedural dissection, perforation, embolism, thrombosis, compartment syndrome, major amputation, emergent vascular surgery, or major bleeding was 5.1% (n=1,565) and was not significantly different between patients treated with atherectomy (4.8%, n=524) compared with patients treated with POBA±stent (5.3%, n=1,041; p=0.07) (Table 2). In multivariable analysis, the use of atherectomy was not associated with an increased risk of the combined IH endpoint (OR 0.98, 95% CI: 0.87-1.10; p=0.68).
Table 2. Unadjusted in-hospital outcomes.
| Atherectomy (n=10,971) | POBA±stent (n=19,876) | p-value | |
|---|---|---|---|
| Composite: procedural dissection, perforation, embolism, thrombosis, compartment syndrome, major amputation, emergent vascular surgery, or major bleeding | 524 (4.8) | 1,041 (5.3) | 0.074 |
| Procedural dissection | 132 (1.2) | 323 (1.6) | <0.01 |
| Perforation | 46 (0.4) | 103 (0.5) | 0.23 |
| Embolism | 58 (0.5) | 67 (0.3) | 0.01 |
| Thrombosis | 55 (0.5) | 107 (0.5) | 0.66 |
| Compartment syndrome | 7 (0.1) | 27 (0.1) | 0.07 |
| Major amputation (unplanned) | 29 (0.3) | 81 (0.4) | 0.04 |
| Emergent vascular surgery | 5 (0.0) | 19 (0.1) | 0.13 |
| Major bleeding (within 72 hrs) | 204 (1.9) | 355 (1.8) | 0.65 |
| Data are n (%). POBA: plain old balloon angioplasty | |||
OUT-OF-HOSPITAL OUTCOMES
Of the study population, 6,889 (22.4%) patients were linkable with Medicare to evaluate out-of-hospital outcomes. Of these 6,889 linked patients, the mean age of the cohort was 74.5±7.7 years, and 41.5% (n=2,859) were female. Overall, in the unadjusted analysis, there was no difference in the cumulative incidence of amputation for atherectomy versus POBA±stent at 1 year (3.7% vs 3.6%; p=0.3). After adjustment, atherectomy use during PVI was associated with a reduced risk of amputation at 1 year compared to non-atherectomy PVI procedures (adjusted HR 0.67, 95% CI: 0.51-0.85; p=0.001). Atherectomy compared with POBA±stent was associated with a reduced rate of surgical revascularisation (1.5% vs 3.1%; p<0.001), an increased rate of endovascular revascularisation of either limb (14.1% vs 11.7%; p=0.003), and there was no difference in death rates (14.1% vs 15.4%; p=0.13). After adjustment, atherectomy was associated with a reduced risk of surgical revascularisation (adjusted HR 0.63, 95% CI: 0.44-0.89; p<0.01), no difference in death rates (adjusted HR 0.90, 95% CI: 0.81-1.02; p=0.10) but an increased risk of endovascular revascularisation (adjusted HR 1.21, 95% CI: 1.06-1.39; p<0.01) at 1 year (Central illustration).
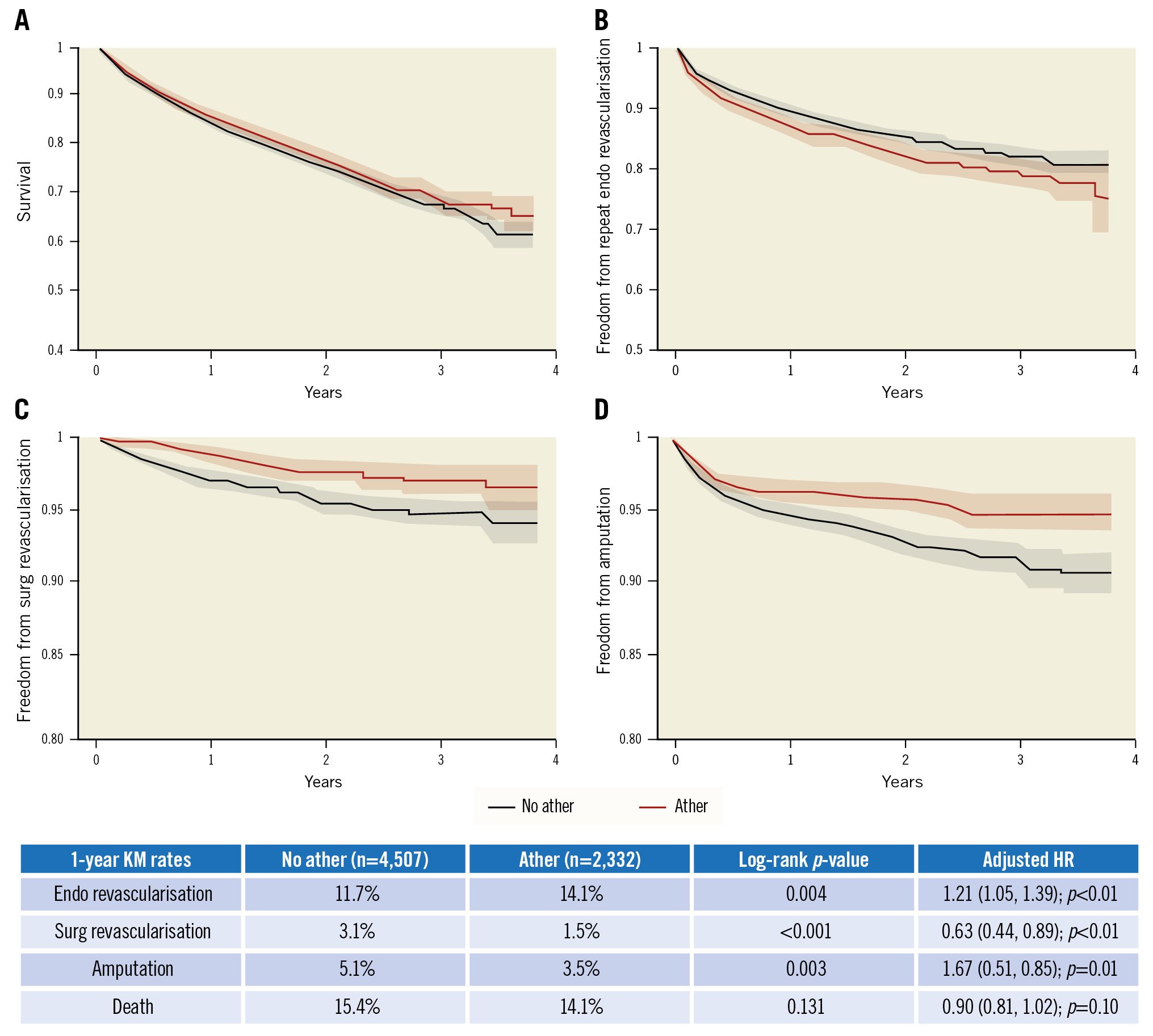
Central illustration. Kaplan-Meier curves of the unadjusted association between atherectomy (ather) versus non-atherectomy (no ather) PVI. A) KM curves for survival, B) KM curves for freedom from endovascular (endo) revascularisation, C) KM curves for freedom from surgical (surg) revascularisation, and D) KM curves for freedom from amputation. Risk-adjusted hazard ratios (HR) for these associations are shown in the accompanying table. Ather: atherectomy; KM: Kaplan-Meier
Discussion
There is a relative paucity of large-scale and real-world data on in-hospital and out-of-hospital outcomes among patients with PAD undergoing PVI with atherectomy. In this large, real-world population of patients with PAD undergoing lower extremity PVI, atherectomy was not associated with an increased risk of procedural-related IH events. In addition, atherectomy was associated with a reduced risk of surgical revascularisation, amputation, and death but an increased risk of endovascular revascularisation at 1-year follow-up.
In one of the largest studies of real-world outcomes following atherectomy, investigators examined over 16,000 patients with PAD undergoing PVI in the Vascular Quality Initiative (VQI) registry which was linked to Medicare12. The study found that atherectomy was associated with increased rates of amputation and major adverse limb events (defined as major amputation or any reintervention) following atherectomy compared to POBA or stenting at 5 years of follow-up. Both major amputation and any reintervention (PVI or surgical bypass) individually occurred at a greater frequency in the atherectomy group. In the current contemporary study of a larger US population including Medicare patients with 1 year of follow-up, we did not observe a higher risk of amputation following atherectomy±stenting compared to POBA±stenting. We observed an increased rate of endovascular revascularisation but a decreased rate of surgical revascularisation following atherectomy.
The primary difference between this study and that by Ramkumar et al was that the latter did not differentiate between types of reintervention, and thus, it is possible that the reduction seen in more invasive and costly surgical reinterventions occurred in the context of a greater increase in endovascular reinterventions12. In contrast to their study, we did not include laser atherectomy, as this form of plaque modification is not commonly used for calcified lesions, and thus, the outcomes associated with non-laser atherectomy may have differed from their reported findings. In addition, we included patients who received stenting following atherectomy in the atherectomy group, as stents are commonly utilised following atherectomy but less so compared to POBA, as observed in the current study. We observed that over 30% of patients underwent stenting and over 50% were treated with a DCB following atherectomy compared to nearly 60% and 25%, respectively, following POBA. Ramkumar et al also did not examine all-cause mortality, which we observed to be similar between the atherectomy and non-atherectomy groups. Lastly, the investigators did not report on in-hospital outcomes following atherectomy, whereas we observed a low rate of serious adverse events overall, particularly among patients treated with atherectomy.
Procedures captured by the VQI registry include those performed at academic medical centres, teaching hospitals, community hospitals, office-based labs and individual physician’s practices. Notably, atherectomy is performed nearly twice as often in office-based labs compared with inpatient and outpatient hospital settings17. The NCDR PVI Registry is an in-hospital registry that only captures inpatient and outpatient procedures done in hospitals and not in office-based labs. Furthermore, the NCDR PVI Registry is more heavily weighted towards interventional cardiologists as opposed to VQI, which is primarily vascular surgeons and other non-cardiology interventionalists. Although both the number of procedures and overall experience of the operator may influence the outcomes of PVI with or without atherectomy, data from the NCDR PVI registry were not available to characterise outcomes by operator speciality. In an analysis of 924 patients enrolled in Medicare Part B from 2012 to 2014 undergoing atherectomy in outpatient hospital-based and office-based laboratory settings, major amputation rates following femoropopliteal atherectomy were 3.2% and 2.3%, respectively, and 8.1% and 5.0% for tibiopedal atherectomy, respectively, at 18 months after the index PVI18. These amputation rates are higher than those observed in the current study or in other smaller studies19 including among patients with claudication or CLI undergoing infrainguinal atherectomy in office-based labs20.
Reduced mortality was observed at 1 year among 218,858 Medicare beneficiaries that included 56,361 individuals undergoing PVI with any type of atherectomy for CLI or claudication from 2010 to 2012, whereas we did not observe any association between atherectomy and mortality in our Medicare-linked analysis of a smaller population17. Consistent with our findings with regard to surgical revascularisation, the investigators reported an increased rate of revascularisation but did not distinguish between the mode of revascularisation (i.e., PVI or surgical revascularisation)17. Additionally, the association between atherectomy and amputation was not reported, but amputation rates were lower in the office-based laboratory setting, where atherectomy was more common17. In another analysis of Medicare beneficiaries with CLI undergoing PVI and atherectomy, Mustapha et al observed reduced mortality and lower amputation rates, as was observed in our study, and an increased rate of reintervention among those treated with atherectomy at 4 years13. Differences in patient population, sample size, and follow-up duration may account for differences in mortality among these studies and our analysis; however, the increased rate of reintervention is broadly consistent with our findings with regard to endovascular reintervention.
Few large-scale data are available regarding the in-hospital rates of adverse events among patients undergoing PVI with atherectomy (5%) compared to POBA±stent (5.3%). We observed a low rate of in-hospital adverse events including bleeding, perforation, or emergency vascular surgery following PVI with atherectomy. In 500 patients with PAD (259 with CLI) treated with directional atherectomy, major complication rates were also reassuringly low at <1% at the time of index PVI21. In 1,204 patients (703 with CLI) treated with orbital atherectomy in the Liberty 360 registry, the rates of significant angiographic complication at the time of index PVI, defined as severe dissection, perforation, abrupt closure or distal embolisation, were 8.5%, 11.7%, and 14.7% among individuals in Rutherford class 2-3, 4-5, and 6, respectively22. The increased rate of adverse events observed in Liberty 360 compared to the current study may be related to increased ascertainment of these events in the context of a prospective, industry-sponsored registry and also a population with greater PAD severity.
Limitations
The current retrospective study has several limitations. Unmeasured confounders may be present in our population that could have influenced treatment selection, such as patient frailty, arterial calcification, procedural risk, and PAD complexity/severity. Treatment strategy also varied between groups, with stent use being less common and drug-coated balloon use more common in the atherectomy group, which may have impacted our findings. Of note, only paclitaxel-coated balloons were included in this analysis, as no other type of drug-coated balloon had approval for use in the USA during the study period. The laterality of revascularisation or amputation is not captured by Medicare data, so it is not possible to know if these events occurred in the same limb that was treated at the time of the index PVI. Reassuringly, the relatively low rate of reported events is consistent with those reported in the literature. In addition, procedural complications and in-hospital outcomes are important metrics of the safety and efficacy of vessel preparation during PVI, and thus insights into the association between vessel preparation, procedural complications, and in-hospital outcomes may impact clinical practice. The possibility of bias due to loss to follow-up cannot be excluded, since <25% of patients were Medicare beneficiaries with available long-term follow-up data.
Conclusions
The use of atherectomy during PVI in a national, contemporary cohort of patients undergoing PVI is associated with a reduced risk of amputation and surgical revascularisation but is associated with an increased rate of endovascular reintervention at 1 year. Furthermore, the use of atherectomy, an important tool in the management of complex PAD, is not associated with an increased risk of mortality. These findings support the short- and long-term safety of atherectomy in patients with PAD undergoing PVI and highlight the need for large outcome-oriented studies to evaluate the impact of atherectomy on limb-specific efficacy endpoints.
Impact on daily practice
Atherectomy is commonly used in patients treated in a hospital-based setting undergoing lower extremity PVI in the USA. Reassuringly, the use of atherectomy is not associated with an increased risk of in-hospital complications and may be associated with favourable outcomes in follow-up. However, further studies are needed to evaluate the impact of atherectomy on long-term outcomes after atherectomy in the context of PVI.
Funding
This study was sponsored by a grant from Cardiovascular Systems, Inc.
Conflict of interest statement
M.S. Albaghdadi reports fees/honoraria for consultancy or participation in a speakers bureau from Genetesis, Cardiovascular Systems, Inc., and Edwards Lifesciences. M. Young reports fees/honoraria for consultancy or participation in a speakers bureau from Medtronic. P. Monteleone reports fees/honoraria for consultancy or participation in a speakers bureau from Boston Scientific, Biotronik, and Medtronic. B. Hawkins reports fees/honoraria for consultancy or participation in a speakers bureau from Baim, CLS Behring, and Hemostemix. E. Armstrong reports fees/honoraria for consultancy or participation in a speakers bureau from Abbott, Boston Scientific, CSI, Gore, Medtronic, Philips, and Shockwave Medical. E. Secemsky reports fees/honoraria for consultancy or participation in a speakers bureau from Abbott, Bayer, Becton, Dickinson and Company, Boston Scientific, Cook, Cardiovascular Systems, Inc., Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and VentureMed. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

