
Abstract
Aims: Peripheral artery disease (PAD) is common in patients with aortic valve stenosis (AS). This study sought to assess the prevalence of critical limb ischaemia (CLI) and its impact on in-hospital outcome in patients undergoing transcatheter aortic valve implantation (TAVI) for severe AS.
Methods and results: All isolated TAVI procedures for AS in Germany between 2007 and 2013 were analysed regarding the stage-specific prevalence of PAD, comorbidities, in-hospital complications and mortality using diagnostic and procedural codes. Among 32,044 patients with TAVI, 3,375 (10.5%) had PAD and 654 (2.0%) CLI. TAVI patients with PAD, particularly those with CLI, had a higher incidence of periprocedural stroke, bleeding and acute kidney injury (p<0.001). The overall in-hospital mortality among TAVI without PAD, non-CLI PAD and CLI was 6.1%, 8.4% and 14.7%, respectively (p<0.001). In a multivariate logistic regression analysis, CLI was an independent predictor of in-hospital mortality (odds ratio 1.96, 95% confidence interval 1.56-2.47; p<0.001).
Conclusions: In patients undergoing TAVI, the presence of PAD is associated with an increased risk of periprocedural complications, while only CLI independently predicts increased in-hospital mortality. Whether CLI represents a marker of general poor health status resulting in poor outcome or is a modifiable risk factor whose treatment prior to TAVI can improve the outcome requires prospective studies.
Abbreviations
AKI: acute kidney injury
CLI: critical limb ischaemia
EuroSCORE: European System for Cardiac Operative Risk Evaluation
ICD-10-GM: International Statistical Classification of Diseases and Related Health Problems 10th Revision
LOS: length of stay
OPS: German procedure classification system
PAD: peripheral artery disease
PPI: permanent pacemaker implantation
TAVI: transcatheter aortic valve implantation
Introduction
In the last decade, transcatheter aortic valve implantation (TAVI) has evolved as a minimally invasive technique and has become the therapy of choice for the treatment of symptomatic, severe aortic valve stenosis in patients with a high perioperative risk1-3. Peripheral artery disease (PAD) shares the underlying risk factors of aortic valve stenosis and is therefore common in these patients. The presence of PAD – irrespective of concomitant disease and studied population – impacts adversely on short- and long-term outcome in terms of periprocedural complications and mortality4. Previously published studies have reported the detrimental impact of PAD on outcome in TAVI patients5, indicating that PAD may worsen the outcome beyond the effect of PAD-related access-site complications. Those studies reported PAD in TAVI in a dichotomous pattern, i.e., either presence or absence without any relation to the severity of PAD. The impact of PAD on short- and long-term outcome, however, is stage-specific, i.e., complications and mortality are correlated with the severity of PAD, with the highest rates among those classified as having critical limb ischaemia (CLI)6.
This study sought to determine the prevalence and impact of stage-specific PAD on in-hospital complications and mortality in patients undergoing TAVI in Germany.
Methods
Data of all isolated TAVI performed for severe, symptomatic aortic valve stenosis in Germany between 2007 and 2013 were retrieved and analysed. Analyses were performed on behalf of the authors by the Research Data Centres of the Federal Bureau of Statistics (Statistisches Bundesamt, DESTATIS; https://www.destatis.de), and aggregated data returned to the authors. Since neither the investigators nor the personnel of the Research Data Centres of the Federal Bureau of Statistics had access to individual patient data but only to summary results, the approval of the ethics committee was not required in accordance with German law.
To account for the learning curve effect on outcome trend, we divided the entire data set into two cohorts: the initial phase where TAVI techniques and procedures were evolving (2007-2010 cohort), and the later period (2011-2013 cohort) where TAVI technique had been established.
DIAGNOSES AND PROCEDURE CODES
The details of the methodology of data source and of data acquisition have been described previously3. All diagnoses are coded according to the German Modification of the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10-GM). Similar to the ICD codes, all diagnostic and therapeutic procedures, including endovascular and surgical interventions, are coded according to the German procedure classification system (OPS). We used these OPS codes for TAVI (OPS codes: in 2007 for transfemoral and transapical 5-35 a.0; from 2008 onwards 5-35 a.00 for transfemoral and 5-35 a.01 for transapical TAVI) to identify all hospitalisation cases with isolated TAVI. Concomitant PAD of the lower extremities at distinct stages according to the Fontaine classification7 was obtained from the ICD-10-code (I70.20-I70.24). Of note, due to revision of the ICD codes for PAD in 2015, the currently used codes for classification of PAD are different from the codes used at the time of the data acquisition.
Comorbidities and complications were also assessed from the diagnostic and interventional procedure codes. Previous cardiac surgery indicates a history of previous coronary bypass or valve surgery. Bleeding was defined as requiring more than five units of red blood cells during the hospital stay. The logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE; www.euroscore.org/euroscore_scoring.htm) was calculated using the ICD-10-GM codes and the patients’ characteristics, as described previously3.
STATISTICAL ANALYSIS
The entire TAVI cohort was divided into three groups: those without a concomitant diagnosis of PAD (non-PAD), those having PAD identified by ICD-10 codes I70.20-I70.21 (asymptomatic and claudicants=PAD at non-CLI), and patients with ICD-10 codes I70.22-I70.24 (having rest pain and/or tissue loss, i.e., ulcer/gangrene=PAD at CLI). Differences in baseline parameters between groups and within groups over time were calculated using the chi-square test for categorical variables and the Student’s t-test for continuous variables. Multivariate logistic regression analyses were used to outline the impact of the distinct stages of PAD and other baseline risks (expressed by the estimated logistic EuroSCORE) on in-hospital mortality. Regression analyses were performed using Stata, version 13 (StataCorp, College Station, TX, USA).
Results
PATIENT CHARACTERISTICS
Data of 32,044 isolated TAVI procedures performed between January 2007 and December 2013 in Germany were analysed. The baseline characteristics of these TAVI procedures are presented in Table 1.
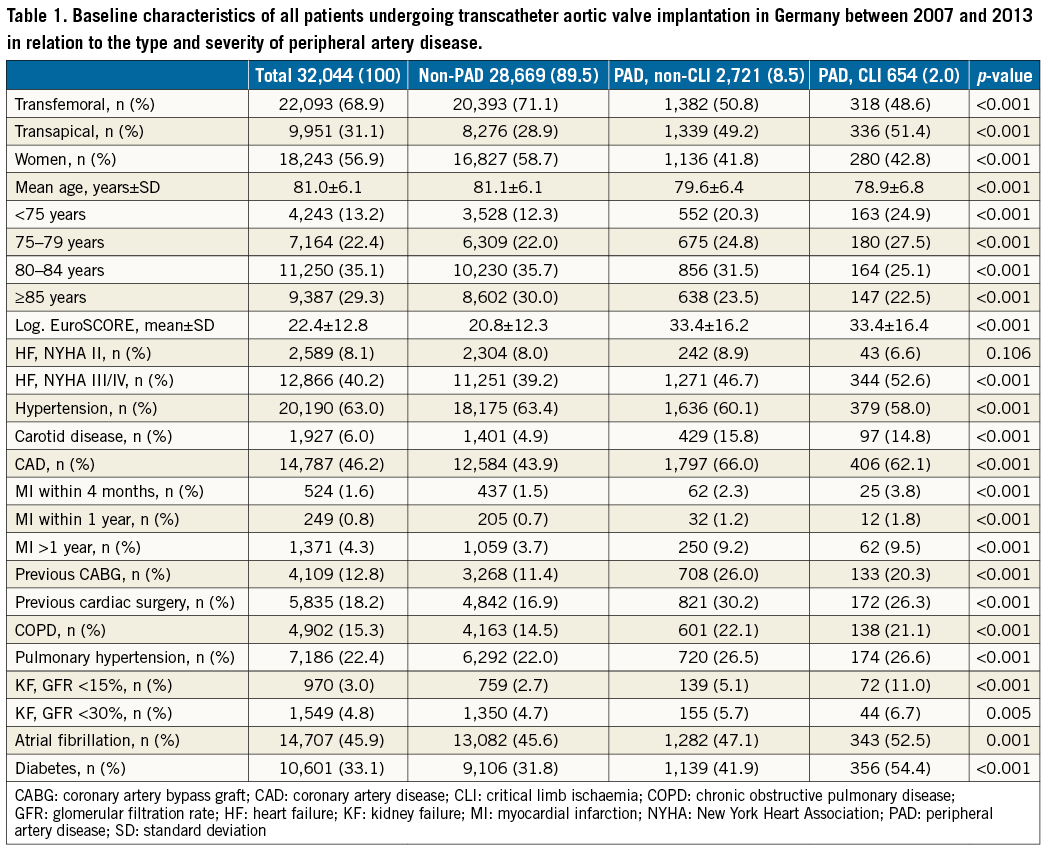
Of the entire TAVI cohort, 3,375 (10.5%) patients had concomitant PAD: 2,721 (80.6%) of these were classified as non-CLI PAD and 654 (19.4%) were classified as CLI. Transfemoral and transapical access was used in 22,093 (68.9%) and in 9,951 (31.1%) TAVI cases, respectively.
Patients undergoing TAVI without PAD were older and more likely to be female than those with PAD (Table 1). In contrast, patients with PAD had higher frequencies of concomitant comorbidities, resulting in a higher estimated logistic EuroSCORE in PAD compared to non-PAD patients (33±16% vs. 21±12%, p<0.001). However, the estimated logistic EuroSCORE was not different within the PAD cohort between non-CLI and CLI patients.
IN-HOSPITAL COMPLICATIONS AND MORTALITY IN RELATION TO PAD STATUS
The rate of TAVI-related in-hospital complications and overall in-hospital mortality for the time periods 2007-2010 and 2011-2013 are presented in Figure 1A and Figure 1B.
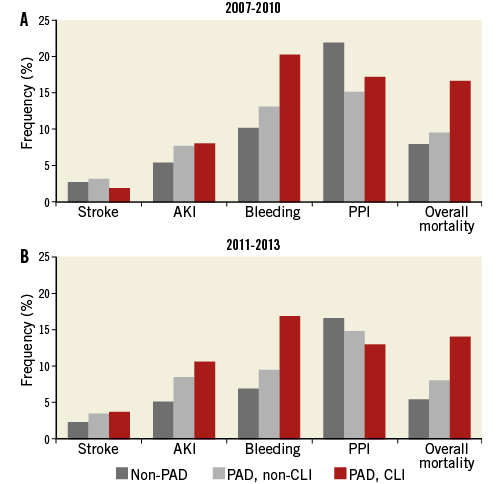
Figure 1. In-hospital complications among patients undergoing transcatheter aortic valve implantation. Frequencies of in-hospital complications among patients undergoing transcatheter aortic valve implantation in 2007-2010 (A) and 2011-2013 (B). AKI: acute kidney injury; CLI: critical limb ischaemia; PAD: peripheral artery disease; PPI: permanent pacemaker implantation
Permanent pacemaker implantation (PPI) was the most frequent complication in TAVI, followed by bleeding, acute kidney injury (AKI) and stroke. Except for PPI, which occurred more often in non-PAD patients (18%), all other complications were more frequent in patients with PAD, with the highest rates among CLI patients. Regarding temporal changes (2007-2010 vs. 2011-2013) in the non-PAD patients, stroke (2.6% vs. 2.3%), bleeding (10.1% vs. 6.9%) and PPI (21.8% vs. 16.6%) significantly decreased (p<0.001), while AKI remained constant (p=0.755). In the PAD, non-CLI subset, the TAVI-related complication rates for stroke (3.1% vs. 3.4%), AKI (7.6% vs. 8.4%) and PPI (15% vs. 14.9%) remained unchanged, while bleeding (13.1% vs. 9.6%) decreased significantly (p=0.009). In CLI patients, stroke (1.8% vs. 3.7%) and AKI (8.0% vs. 10.6%) increased, while bleeding (20.2% vs. 16.9%) and PPI (17.2% vs. 13.0%) decreased (p<0.001). The overall mortality decreased in all groups over time (p<0.001) (Figure 1A, Figure 1B).
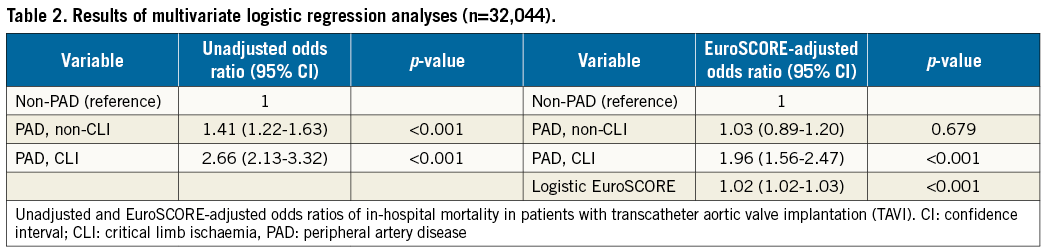
In the unadjusted logistic regression analysis, the odds of in-hospital mortality were substantially increased for non-CLI and CLI PAD patients (odds ratio [OR] 1.41, 95% confidence interval [CI]: 1.22-1.63, p<0.001, and OR 2.66, 95% CI: 2.13-3.32, p<0.001, respectively) (Table 2) compared to non-PAD patients. Regarding temporal changes, there was a slight increase in the OR for in-hospital mortality in the later “established” cohort (OR 2.84, 95% CI: 2.19-3.69) compared to the earlier “evolving” cohort (OR 2.34, 95% CI: 1.53-3.56). After further adjustment for the estimated logistic EuroSCORE, however, only CLI patients were associated with increased odds of in-hospital mortality (OR 1.96, 95% CI: 1.56-2.47, p<0.001).
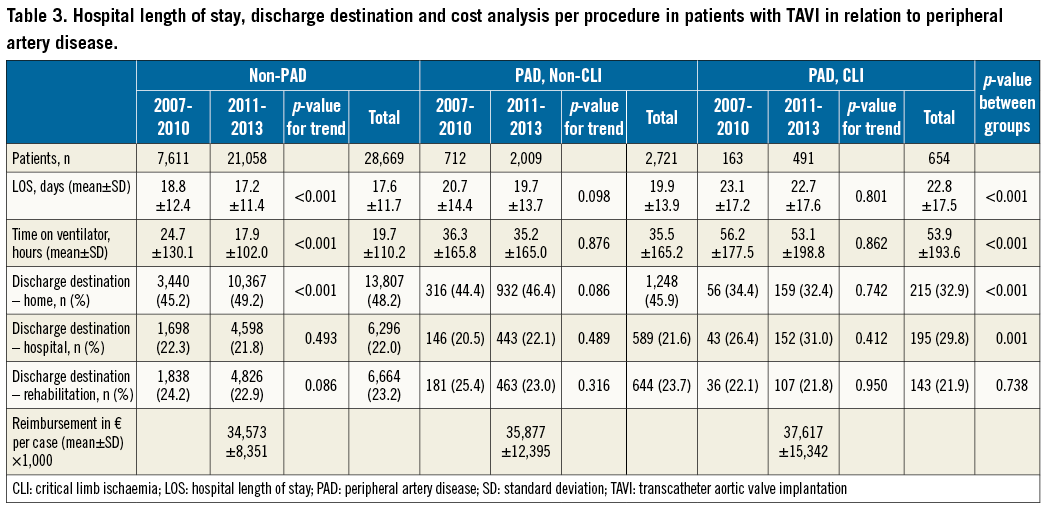
LENGTH OF HOSPITAL STAY, DISCHARGE AND COST ANALYSIS IN RELATION TO PAD
Resource utilisation indices such as the in-hospital length of stay (LOS), perioperative and postoperative ventilation time and another hospital as a discharge destination were highest for CLI PAD and lowest for non-PAD patients (p<0.001) (Table 3). The resulting total reimbursement costs (in 1,000 €) per TAVI case were accordingly highest for patients with CLI PAD (37.6±15.3 €) followed by non-CLI PAD (35.9±12.4 €) and lowest for patients without PAD (34.6±8.4 €, p<0.01).
Discussion
PAD as a marker of severe, general atherosclerosis has been shown to impact adversely on the outcome in any population, irrespective of the underlying and concomitant diseases8. In this context, the finding of higher complication rates and in-hospital mortality rates in PAD compared to non-PAD patients undergoing TAVI in this study is not surprising. What is new, however, is the finding that not PAD in general, but the progressive stage of CLI is a strong and logistic EuroSCORE-independent predictor of increased in-hospital mortality in patients undergoing TAVI.
The adverse impact of PAD in general on outcome in TAVI patients has previously been demonstrated in selected and smaller cohorts5. Moreover, in all published studies dealing with PAD in TAVI, PAD was generally considered as a categorical entity, i.e., either the presence or absence of PAD without any relation to its distinct clinical stages. The incremental prognostic value of PAD on outcome parameters such as complications and mortality, however, is strongly related to the clinical stage of PAD. Large-scale, population-based studies have demonstrated an up to fivefold higher in-hospital mortality in CLI patients compared to claudicants9. Ischaemic tissue loss of extremities indicates a status of severe, generalised and decompensated atherosclerosis and is associated with extensive resource utilisation, complications and high mortality rates.
PREVALENCE OF PAD AND CLI IN PATIENTS WITH TAVI
This is the first study reporting the prevalence and outcome implications of CLI in patients undergoing TAVI. The 12% prevalence of PAD in octogenarians with severe calcific aortic valve stenosis is certainly underestimated due to the fact that no systematic diagnostic approach using different complementary modalities such as measurement of ankle-brachial index, oscillography and duplex ultrasound was used to assess PAD. The prevalence of PAD is highly correlated to age and the burden of cardiovascular risk factors. Epidemiological studies have revealed the prevalence of PAD as being only 3-5% in a general population aged <50 years and without risk factors, but increasing to 29% in those having multiple risk factors10 and to 38% in patients with manifest atherosclerosis in other vascular territories11. The German Aortic Valve Replacement Registry (GARY) reported a PAD prevalence of 19.5% in patients undergoing TAVI12. Compared to the present study, concomitant PAD in TAVI cohorts has been reported at higher rates in previous studies, such as in US TAVI (32.4%)13, the French Transcatheter Aortic Valve Intervention Registry (FRANCE 2 Registry, 20.8%)14, and the United Kingdom Transcatheter Aortic Valve Implantation Registry (26.5%)15.
IN-HOSPITAL OUTCOME IN RELATION TO PAD
While the presence of PAD in TAVI was associated with a higher incidence of periprocedural complications compared to non-PAD patients, CLI particularly predicted increased in-hospital mortality: it was lowest among non-PAD patients (6.1%), intermediate in non-CLI PAD (8.4%), and the highest mortality rate was observed in CLI PAD patients (14.7%). In the fully adjusted model (Table 2), non-CLI PAD did not significantly impact on in-hospital mortality while CLI significantly increased the risk for in-hospital mortality. Furthermore, the increase of mortality attributed to CLI was independent from common cardiovascular risk factors covered by the logistic EuroSCORE, as shown by the multivariate logistic regression analysis. The logistic EuroSCORE, which was originally designed to estimate the perioperative mortality in patients undergoing cardiac surgery, is currently the recommended score for estimation of mortality in TAVI patients. However, the logistic EuroSCORE does not discriminate between PAD and CLI or other manifestations of peripheral atherosclerosis but these are rather unspecifically allocated to the term “extracardiac arteriopathy”. Moreover, the logistic EuroSCORE does not cover all conditions and factors contributing to or determining the overall in-hospital mortality following TAVI. As CLI represents a poor general medical condition prone to adverse outcome, it is conceivable that CLI patients had more frail conditions not covered and hence not detected by the logistic EuroSCORE, such as infections, inflammation, uncontrolled status of cardiovascular risk factors, etc., which might have resulted in the observed increased mortality. This assumption is supported by the significantly higher resource utilisation parameters such as length of hospital stay, ventilation time, another hospital as discharge destination and the total reimbursement costs in CLI compared to non-CLI and non-PAD patients (Table 3). Our data suggest, therefore, that CLI rather than general PAD should be an integral part of the preprocedural risk stratification. Consequently, distinction between CLI and non-CLI PAD is important when assessing the preoperative risk of patients destined for TAVI.
Consistent with previous observations16, the overall complication rate in TAVI in our analysis has decreased over time, mainly driven by a reduction in bleeding and the decreased need for PPI. However, in the subset of TAVI patients with PAD, particularly in CLI patients, the incidence of stroke and AKI conversely increased. A combination of several factors such as a more judicious patient selection, advances in dedicated devices, decreasing diameters of delivery systems, enhanced technical skills and a better management of procedure-related complications might have contributed to the reduction in the overall complication rate. The adverse trend in stroke and AKI in PAD patients, on the other hand, might mirror the continuously increasing risk profile of PAD, particularly of CLI patients9. In particular, pre-existing chronic kidney disease has been shown to be an independent predictor of postinterventional AKI following TAVI17.
Limitations
As with any studies which are based on administrative data, our analysis is prone to possible coding errors which is a major flaw of the study. Therefore, the prevalence of PAD, of comorbidities and of calculated scores such as the logistic EuroSCORE might be inaccurate. The majority of PAD patients are asymptomatic and even symptoms which are present are frequently unspecific and unreliable. Moreover, in the context of pre-TAVI work-up, there was no systematic use of complementary diagnostic tools such as ankle-brachial index and toe-brachial index, oscillography and thorough duplex ultrasound, which have been shown to be useful and to enhance the detection rate of PAD. Therefore, the prevalence of non-CLI PAD in the TAVI population in this study is certainly underestimated. In contrast, the prevalence of CLI should be accurate since it is simple to define and detect (rest pain and/or ulceration, gangrene), and therefore less susceptible to under-reporting and miscoding.
Since the analysis is based on administrative data, we can only report the in-hospital outcome measures. The impact of PAD, and particularly of CLI, on midterm and long-term outcome in TAVI remains to be determined. Though large-scale, covering the data set of an entire nation over seven years, our analysis is restricted to the TAVI population in Germany. The prevalence and impact of PAD and CLI on outcome in TAVI might differ in other populations.
Conclusions
Despite the above-mentioned limitations, this study is the first to elaborate the prevalence of CLI and its detrimental impact on in-hospital complications and mortality in patients with TAVI in a real-world database of an entire nation. Whether CLI represents a marker of general poor health status resulting in poor outcome or a modifiable risk factor, the treatment of which prior to TAVI can improve the outcome, requires prospective studies.
| Impact on daily practice Peripheral artery disease (PAD) is common in patients undergoing transcatheter aortic valve implantation (TAVI). The extent and severity of PAD not only impacts on the access site decision but also on the in-hospital complication rate and outcome. Therefore, the pre-interventional assessment of the severity of concomitant PAD is crucial, as demonstrated by this analysis. However, whether CLI represents a marker of general poor health status resulting in poor outcome or a modifiable risk factor, the treatment of which prior to TAVI can improve the outcome, requires prospective studies. |
Acknowledgements
We thank Mrs Susanne Schüler and Dr Christiane Engelbertz, both from the Division of Vascular Medicine, Department of Cardiovascular Medicine, University Hospital Muenster, for their excellent assistance during manuscript preparation.
Funding
The study was funded by the Heart Center, Freiburg University, Freiburg, Germany.
Conflict of interest statement
N. Malyar has received travel support from Bard, Bayer Vital, Cordis, and Medtronic, and speaker honoraria from Daiichi Sankyo, Medac, and UCB Pharma. E. Freisinger has received travel support from Bard, and DGA German Society of Vascular Medicine. G. Kaleschke has received speaker fees from Edwards Lifesciences. H. Baumgartner has received speaker honoraria and travel support from Edwards Lifesciences and travel support from St. Jude Medical, Abbott, Medtronic, Gore, and Direct Flow Medical. H. Reinecke has received speaker honoraria from Sanofi-Aventis, Daiichi Sankyo, The Medicines Company, Cordis, BMS, and Novartis. He has acted as a consultant for BMS and Pluristem. He took part in the conduct of multicentre trials of Bard, Bayer, Biotronik, and Pluristem. J. Reinöhl has received research grants and personal fees form Edwards Lifesciences and consultant fees from Direct Flow Medical and JenaValve. The other authors have no conflicts of interest to declare.

