Abstract
Background: It remains unclear whether the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria could apply to peripheral artery disease (PAD) patients undergoing endovascular therapy (EVT).
Aims: We sought to evaluate the application of the ARC-HBR criteria to PAD patients undergoing EVT with contemporary drug-coated devices (DCD) for femoropopliteal artery lesions.
Methods: Between May 2012 and December 2019, 542 consecutive patients undergoing EVT with DCD for femoropopliteal artery lesions were retrospectively analysed. The primary study endpoint was major bleeding events, defined as Bleeding Academic Research Consortium type 3 or 5.
Results: Of 542 patients, 435 (80.3%) were stratified into the HBR group. The cumulative 5-year incidence of major bleeding events was significantly higher in the HBR group than in the non-HBR group (31.9% vs 2.3%; p<0.001). The 5-year major bleeding event rate gradually increased with the number of ARC-HBR criteria (≥2 major criteria: 48.6%, 1 major: 33.1%, ≥2 minor: 12.9%, and non-HBR: 2.3%; p<0.001). Major bleeding events were associated with a 5.4-fold increased risk of mortality (adjusted hazard ratio: 5.42, 95% confidence interval: 2.91-10.1; p<0.001). Severe chronic kidney disease, heart failure, and severe anaemia were predictors of major bleeding events.
Conclusions: 80.3% of PAD patients undergoing EVT for femoropopliteal artery lesions with contemporary drug-coated devices met the ARC-HBR criteria. Given that major bleeding events remarkably increased the risk of mortality after EVT, the ARC-HBR criteria might be helpful for the risk stratification of PAD patients who undergo EVT with contemporary DCD.
Introduction
Among patients with coronary artery disease (CAD) requiring percutaneous coronary intervention (PCI), the identification and management of patients at high bleeding risk (HBR) is of utmost importance. However, there is a paucity of standardisation in defining these populations. Recently, the Academic Research Consortium for HBR (ARC-HBR) proposed consensus-based criteria for patients with HBR undergoing PCI1. Several studies have applied the ARC-HBR criteria to a real-world population and demonstrated the validity and effectiveness of these criteria in clinical practice23.
Peripheral artery disease (PAD) is a manifestation of systemic atherosclerotic disease and has emerged as a serious global health issue in an ageing society. Endovascular therapy (EVT) has been widely accepted for the treatment of PAD and has shown better outcomes with improved drug-coated balloons and stents compared to conventional devices45. Since PAD patients often have several comorbidities (e.g., advanced age, anaemia, or chronic kidney disease [CKD])6, they may be more likely to have HBR. To date, however, in contemporary clinical practice few data are available regarding the prevalence of HBR in patients with PAD. Also, it remains undetermined whether HBR would adversely impact outcomes after EVT, although this has already been established for patients undergoing PCI. In the present study, we sought to assess the prevalence and clinical impact of HBR in PAD patients undergoing EVT for femoropopliteal artery lesions with contemporary drug-coated devices (DCD).
Methods
Study population
From June 2012 to December 2019, a total of 1,428 consecutive patients (1,882 limbs) underwent EVT for femoropopliteal artery lesions in our institution. Of these, we excluded patients who had undergone plain balloon angioplasty alone, bare metal stent implantation, stent graft use, and and other variations with non-DCD to assess the clinical outcomes after EVT with contemporary devices. According to the ARC-HBR criteria, eligible patients were divided into the HBR and non-HBR groups1. This study protocol was approved by the ethics committee at our institution and was in accordance with the Declaration of Helsinki. Written informed consent was waived due to the retrospective study design.
Procedure and follow-up protocol
EVT procedures were performed according to the standard technique45. Dual antiplatelet therapy (DAPT) (aspirin 100 mg/day plus clopidogrel 75 mg/day or cilostazol 100 mg twice a day) ≥2 days before the procedure was recommended. Predilatation was performed with an appropriately sized angioplasty balloon. The distal reference diameter was measured, and an equivalent balloon size was selected. After successful balloon angioplasty, patients were treated with a drug-coated balloon (DCB). The type of DCB, balloon inflation time, and pressure were left to the operator’s discretion. A drug-eluting stent (DES) that was approximately 1 mm larger than the vessel diameter was implanted. All procedures were performed with intravascular ultrasound (IVUS) guidance. The choice of DES (Zilver PTX, Cook Medical; Eluvia, Boston Scientific) was left to operator discretion. After stenting, routine post-balloon angioplasty was performed to achieve better stent expansion and apposition. Regarding the duration of DAPT, at least 1 month was recommended in patients receiving a DCB, but those who received a DES were encouraged to adhere to the product package recommendations (Zilver PTX: at least 2 months; Eluvia: at least 2 to 3 months).
Clinical follow-up was performed with hospital visits within the first month of the procedure and thereafter at least every six months. Clinical examinations, the ankle-brachial index (ABI) measurement, and duplex ultrasonography scans were performed at each visit. The need for repeat revascularisation was based on clinical symptoms, duplex ultrasonography scans, or angiographic findings7.
Endpoints and definitions
The primary study endpoint was the cumulative incidences of major bleeding during the follow-up. Major bleeding was defined as Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding8. The secondary study endpoints included major adverse cardiovascular events (MACE) and major adverse limb events (MALE). MACE was defined as a composite of all-cause mortality, stroke, and myocardial infarction (MI). MALE was defined as a composite of major amputation and any reintervention (either endovascular or surgical). Non-fatal MI was assessed according to the fourth universal definition of MI9. Stroke was defined as an ischaemic or haemorrhagic event necessitating hospitalisation with symptoms lasting >24 hours. Major amputation was defined as any above-ankle amputation. Reintervention was performed for ≥50% stenosis or >2.4 of peak systolic velocity ratio by duplex ultrasonography scan or the recurrence of stenosis ≥50% of the arterial diameter, as determined by angiography, with recurrent clinical symptoms7. The severity of calcification was assessed according to the Peripheral Arterial Calcium Scoring System (PACSS)10. Severe CKD and severe anaemia were defined according to the ARC-HBR criteria1.
Statistical analysis
Categorical values are presented as numbers and percentages and were compared with the chi-squared test. Continuous variables are expressed as mean±standard deviation, or median (lower and upper quartiles). Based on their distribution, continuous variables were compared with the Student’s t-test or the Wilcoxon rank-sum test. The cumulative incidence of study endpoints was estimated by the Kaplan-Meier method, and the difference between the HBR and non-HBR groups was assessed with the log-rank test. The cumulative incidence of major bleeding was assessed according to the number of ARC-HBR major and minor criteria1. Patients with ARC-HBR major criteria were included, according to the number of major criteria, in either the ≥2 major criteria, or 1 major criterion group, regardless of the number of overlapping minor criteria. Patients with ≥2 minor criteria and without any major criteria were included in the ≥2 minor-without-major group. The sensitivity and specificity of the ARC-HBR versus non-ARC-HBR for the occurrence of major bleeding events at 5 years were estimated using the nearest neighbour method to account for censored events11. We further estimated the area under the time-dependent receiver operating characteristics curve (AUC) for 5-year major bleeding events by assigning 0 points to patients with non-ARC-HBR, 1 point to those with ≥2 minor criteria, 2 points to those with 1 major criterion, and 3 points to those with ≥2 major criteria, to assess the discriminative ability at the other cut-off point of the ARC-HBR criteria11.
We used the univariable Cox proportional hazards models for clinical events to estimate hazard ratios (HR) of the HBR group relative to the non-HBR group with 95% confidence intervals (CI). The multivariable Cox model was used to associate major bleeding events with 8 clinically relevant variables (≥75 years, male sex, oral anticoagulant use, severe CKD, severe anaemia, bleeding within 6 months, heart failure, and chronic limb-threatening ischaemia [CLTI])112. To assess the association of major bleeding events during follow-up with all-cause death, we used the univariable and multivariable Cox regression models with a major bleeding event as a time-dependent covariate: once major bleeding events occurred, the indicator for major bleeding events was turned on for the remainder of the follow-up. Adjustment variables were based on clinically relevant factors, which are listed in Table 1. The survival probabilities before and after time-dependent major bleeding events were visualised using the Simon-Makuch method and compared with the Mantel-Byar test.
All statistical analyses were performed by 2 physicians (Y. Tomoi and S. Kuramitsu) and a statistician (T. Shinozaki) with JMP 13.0 software (SAS Institute) and R software version 3.5.2 (R Foundation for Statistical Computing). A p value of <0.05 was considered statistically significant.
Table 1. Baseline clinical characteristics.
| Overall (n=542) |
HBR (n=435) |
Non-HBR (n=107) |
p-value | ||
|---|---|---|---|---|---|
| Age, years | 74.9±8.8 | 76.1±8.7 | 70.1±7.3 | <0.001 | |
| ≥75 years* | 292 (53.9) | 269 (61.8) | 23 (21.5) | <0.001 | |
| Male sex* | 352 (64.9) | 282 (64.8) | 70 (65.4) | 1.00 | |
| Body mass index, kg/m2 | 22.5±3.6 | 22.4±3.6 | 23.3±3.4 | 0.01 | |
| <22.0 kg/m2* | 245 (45.2) | 205 (47.1) | 40 (37.4) | 0.08 | |
| Hypertension* | 483 (89.1) | 394 (90.6) | 89 (83.2) | 0.04 | |
| Dyslipidaemia* | 366 (67.5) | 274 (63.0) | 92 (86.0) | <0.001 | |
| Diabetes mellitus* | 310 (57.2) | 238 (54.7) | 72 (67.3) | 0.02 | |
| Current smoker* | 103 (19.0) | 64 (14.7) | 39 (36.4) | <0.001 | |
| Chronic kidney disease | 388 (71.5) | 366 (84.1) | 22 (20.6) | <0.001 | |
| Haemodialysis* | 161 (29.7) | 161 (37.0) | 0 (0) | <0.001 | |
| Prior PCI* | 265 (48.9) | 216 (49.7) | 49 (45.8) | 0.52 | |
| Prior myocardial infarction* | 67 (12.4) | 55 (12.7) | 12 (11.2) | 0.75 | |
| Prior heart failure* | 85 (15.7) | 74 (17.0) | 11 (10.3) | 0.10 | |
| Prior cerebrovascular disease* | 121 (22.3) | 114 (26.2) | 7 (6.5) | <0.001 | |
| Haemoglobin, g/dl | 12.2±1.9 | 11.7±1.7 | 13.9±1.4 | <0.001 | |
| <11 g/dl* | 145 (26.8) | 145 (33.3) | 0 (0.0) | <0.001 | |
| Platelet, ×104/µl | 20.4±6.7 | 20.0±6.7 | 22.0±6.1 | 0.003 | |
| <10×104/μl* | 11 (2.0) | 11 (2.5) | 0 (0.0) | 0.13 | |
| eGFR, ml/min/1.73 m2 | 40.9±28.3 | 33.8±26.0 | 69.7±16.5 | <0.001 | |
| <30 ml/min/1.73 m2 without haemodialysis* | 53 (9.8) | 53 (12.2) | 0 (0.0) | <0.001 | |
| Rutherford classification | Category 1 | 11 (2.0) | 9 (2.1) | 2 (1.9) | <0.001 |
| Category 2 | 218 (40.2) | 161 (37.0) | 57 (53.3) | ||
| Category 3 | 151 (27.9) | 117 (26.9) | 34 (31.8) | ||
| Category 4 | 51 (9.4) | 47 (10.8) | 4 (3.7) | ||
| Category 5 | 105 (19.4) | 96 (22.1) | 9 (8.4) | ||
| Category 6 | 6 (1.1) | 5 (1.2) | 1 (1.0) | ||
| CLTI* | 162 (29.9) | 148 (34.0) | 14 (13.1) | <0.001 | |
| Medication at discharge | |||||
| Aspirin | 457 (84.3) | 358 (82.3) | 99 (92.5) | 0.01 | |
| Thienopyridine | 513 (94.7) | 411 (94.5) | 102 (95.3) | 1.00 | |
| Cilostazol | 102 (18.8) | 83 (19.1) | 19 (17.8) | 0.78 | |
| Dual antiplatelet therapy* | 431 (79.5) | 337 (77.5) | 94 (87.9) | 0.02 | |
| Oral anticoagulant* | 87 (16) | 87 (20) | 0 (0) | <0.001 | |
| Warfarin | 52 (9.6) | 52 (12.0) | 0 (0) | <0.001 | |
| Direct oral anticoagulant | 36 (6.6) | 36 (8.3) | 0 (0) | <0.001 | |
| Statin | 324 (59.8) | 246 (56.6) | 78 (72.9) | 0.002 | |
| ACEI/ARB | 307 (56.6) | 242 (55.6) | 65 (60.8) | 0.38 | |
| β-blockers | 190 (35.1) | 165 (37.9) | 25 (23.4) | 0.005 | |
| Oral hypoglycaemic agent | 226 (41.7) | 165 (37.9) | 61 (57.0) | <0.001 | |
| Insulin | 102 (18.8) | 80 (18.4) | 22 (20.6) | 0.58 | |
| Data are expressed as mean±standard deviation or number (percentage). *Variables used for the multivariable analysis assessing the association of major bleeding events with all-cause death. Chronic kidney disease defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2.ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CLTI: chronic limb-threatening ischemia; eGFR: estimated glomerular filtration rate; HBR: high bleeding risk; PCI: percutaneous coronary intervention | |||||
Results
Study population and prevalence of HBR
Among the 1,428 patients, 886 were excluded for the following reasons: plain balloon angioplasty alone (n=442), bare metal stent implantation (n=384), stent graft use (n=47), and no DCD use (n=13). Finally, 542 patients were analysed in the present study. Of these, 435 (80.3%) and 107 (19.7%) patients were classified into the HBR and non-HBR groups, respectively. The numbers and proportions of patients with ARC-HBR major and minor criteria are shown in Figure 1.
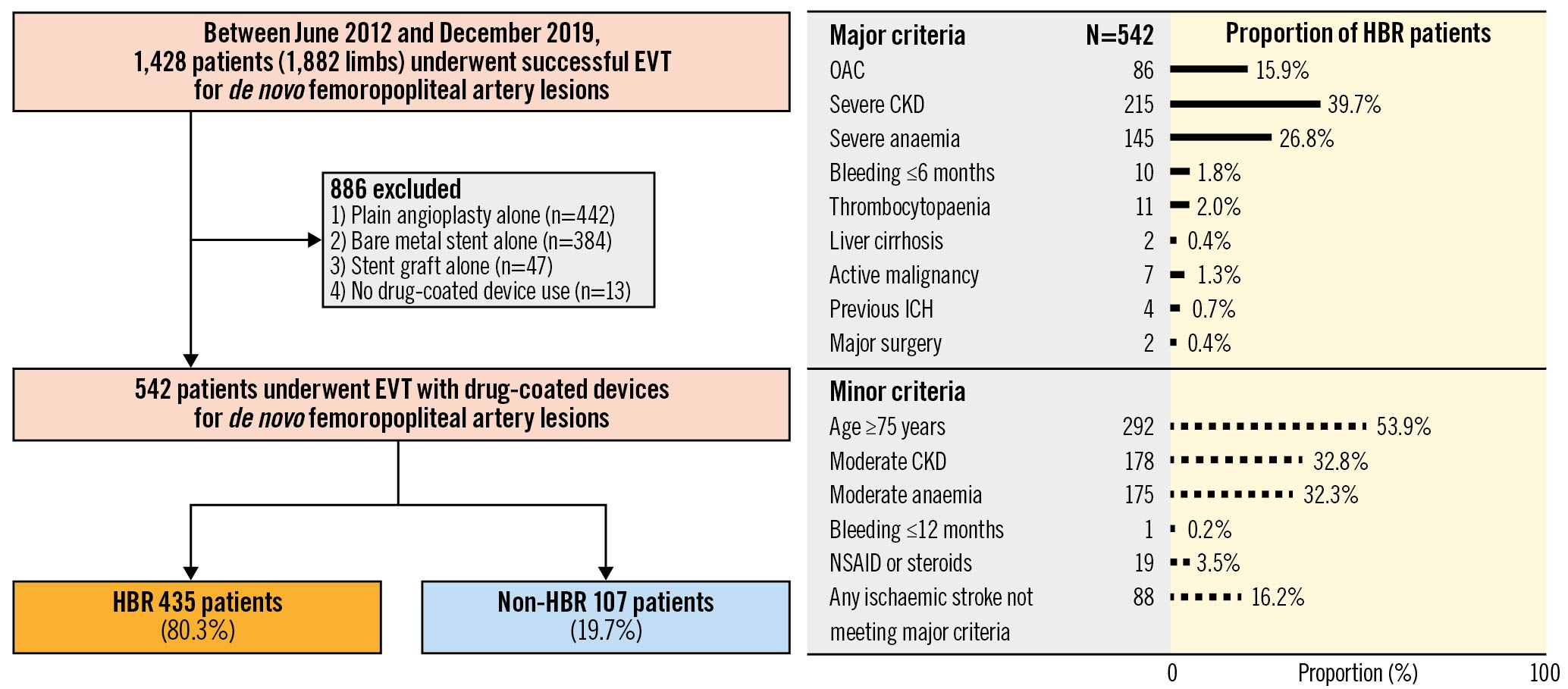
Figure 1. Patient flowchart and proportion of HBR patients by each ARC-HBR criterion. CKD: chronic kidney disease; EVT: endovascular therapy; HBR: high bleeding risk; ICH: intracranial haemorrhage; NSAID: non-steroidal anti-inflammatory drug; OAC: oral anticoagulant
Baseline clinical characteristics
The baseline clinical characteristics are provided in Table 1 and Table 2. Compared with the non-HBR group, the HBR group was older and had lower body mass index. Also, the HBR group had a higher prevalence of hypertension, CKD, haemodialysis, prior cerebrovascular disease, and CLTI, and a lower prevalence of current smoking, dyslipidaemia, diabetes mellitus, and statin use. In terms of lesion and procedural characteristics, no significant differences were observed between the 2 groups except for severe calcification (PACSS grade 3 and 4). Details of the procedural data are provided in Supplementary Table 1.
Table 2. Lesion and procedural characteristics.
| Overall (n=542) |
HBR (n=435) |
Non-HBR (n=107) |
p-value | ||
|---|---|---|---|---|---|
| Lesion characteristics | |||||
| Preprocedural ABI | 0.64±0.27 | 0.63±0.28 | 0.68±0.22 | 0.07 | |
| De novo lesion | 486 (89.7) | 391 (89.9) | 95 (88.8) | 0.72 | |
| Chronic total occlusion | 175 (32.3) | 132 (30.3) | 43 (40.2) | 0.06 | |
| Severe calcium (grade 3 and 4) | 209 (38.6) | 181 (41.6) | 28 (26.2) | 0.004 | |
| Below-the-knee run off | 1.3±0.8 | 1.3±0.8 | 1.4±0.7 | 0.29 | |
| PACSS grade | Grade 0 | 191 (35.2) | 135 (31.0) | 56 (52.3) | <0.001 |
| Grade 1 | 120 (22.1) | 101 (23.2) | 19 (17.8) | ||
| Grade 2 | 22 (4.1) | 18 (4.1) | 4 (3.7) | ||
| Grade 3 | 136 (25.1) | 117 (26.9) | 19 (17.8) | ||
| Grade 4 | 73 (13.5) | 64 (14.7) | 9 (8.4) | ||
| Quantitative vascular analysis | Lesion length, mm | 158.1±84.4 | 157.8±83.1 | 159.0±89.8 | 0.90 |
| Reference vessel diameter, mm | 4.9±1.0 | 4.9±1.0 | 5.0±1.0 | 0.44 | |
| Percent diameter stenosis, % | 89.8±11.1 | 89.5±11.1 | 90.0±10.7 | 0.24 | |
| Procedure characteristics | DES | 205 (37.8) | 164 (37.7) | 41 (38.3) | 0.91 |
| DCB | 321 (59.2) | 256 (58.9) | 65 (60.8) | 0.74 | |
| DES with DCB | 4 (0.7) | 3 (0.7) | 1 (0.9) | 0.59 | |
| DCB with bailout stenting | 6 (1.1) | 6 (1.4) | 0 (0) | 0.60 | |
| Other variation | 6 (1.1) | 6 (1.4) | 0 (0) | 0.60 | |
| Device diameter (mm) | 184.2±93.3 | 184.2±92.0 | 184.3±98.8 | 0.99 | |
| Device length (mm) | 5.8±0.7 | 5.8±0.7 | 5.9±0.7 | 0.82 | |
| Data are expressed as mean±standard deviation or number (percentage). Lesion length, reference vessel diameter, and percent diameter stenosis were calculated by quantitative vascular analysis. ABI: ankle-brachial index; DES: drug-eluting stent; DCB: drug-coated balloon; HBR: high bleeding risk; PACSS: peripheral arterial calcium scoring system | |||||
Continuation of dual antiplatelet therapy
Figure 2 shows the proportion of DAPT continuation during the 2-year follow-up. Overall, HBR patients continued DAPT less frequently than non-HBR patients (Figure 2A). Although the proportion of patients on DAPT decreased with time, DAPT was continued more frequently in patients with DES than those with DCB (Figure 2B). The proportion of HBR patients with DES receiving DAPT was consistently lower than for non-HBR patients over the 2-year follow-up (Figure 2C), while this relationship was not observed in those with DCB (Figure 2D).
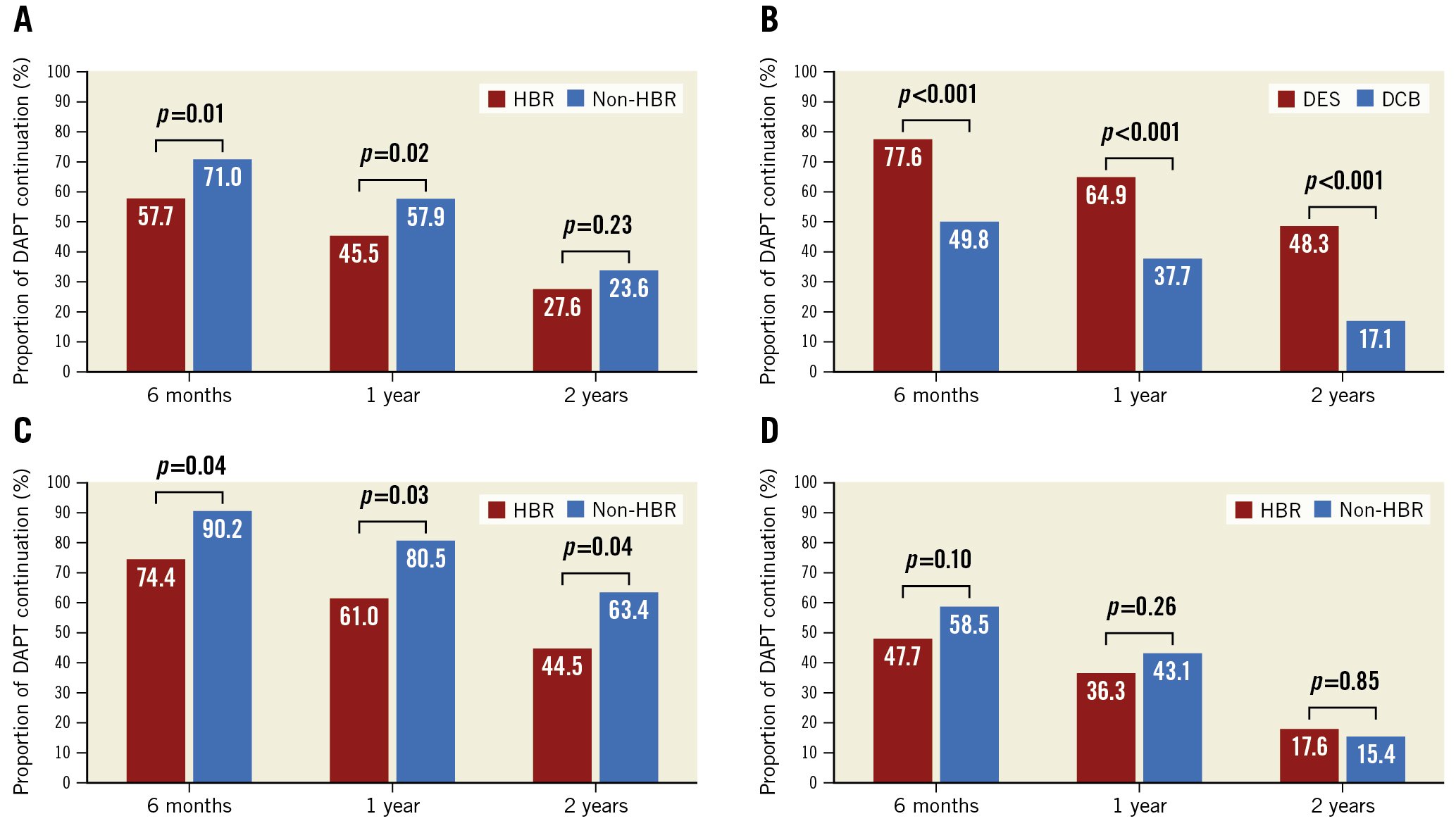
Figure 2. Continuation of dual antiplatelet therapy. (A) Overall, (B) drug-coated devices, (C) drug-eluting stent (DES), and (D) drug-coated balloon (DCB). DAPT: dual antiplatelet therapy; HBR: high bleeding risk
Clinical outcomes
During the median follow-up duration of 2.0 years (1.1 to 2.8 years), the cumulative incidence of major bleeding events was significantly higher in the HBR group than in the non-HBR group (1 year: 11.6% vs 0.9%; 5 years: 31.9% vs 2.3%; p<0.001, respectively) (Table 3, Figure 3). The sensitivity and specificity of the ARC-HBR vs non-ARC-HBR for 5-year major bleeding events were 0.98 and 0.26, respectively (Supplementary Figure 1A). For the 4-category ARC-HBR criteria, the AUC for 5-year major bleeding events was 0.75, with a sensitivity of 0.87 and specificity of 0.53 at the cut-off ≥2 points (1 major or ≥2 major) vs <2 points (≥2 minor or non-HBR) (Supplementary Figure 1B). Major bleeding events were associated with a 5.4-fold increased risk of mortality (adjusted HR 5.42, 95% CI: 2.91-10.1; p<0.001) (Table 4). Figure 4 depicts this increase in hazard after major bleeding events in terms of the survival probability (p<0.001).
The cumulative 5-year incidence of MACE was significantly higher in the HBR group than in the non-HBR group (49.4% vs 17.5%; p<0.001) (Table 3, Figure 5A), mainly driven by all-cause death (Table 3). Also, the cumulative 5-year incidence of MALE was significantly higher in the HBR group than in the non-HBR group (41.4% vs 32.9%; p=0.03) (Table 3, Figure 5B), mainly driven by major amputation and reintervention (Table 3). The 1-year landmark analysis showed that the cumulative incidence of MACE was remarkably higher in the HBR group than in the non-HBR group at and beyond 1 year (15.9% vs 2.9%; p<0.001; 38.8% vs 15.0%; p=0.006, respectively) (Supplementary Figure 2). The cumulative incidence of MALE was significantly higher in the HBR group than in the non-HBR group at 1 year (14.3% vs 4.9%; p=0.01), while no significant difference was observed beyond 1 year (31.6% vs 28.6%; p=0.44) (Supplementary Figure 2). The cumulative 2-year incidence of major bleeding events was higher in the HBR group than in the non-HBR group, regardless of the DCD type (DES with HBR, 16.7%; DCB with HBR, 20.8%; DES with non-HBR, 0.0%; DCB with non-HBR, 4.5%; p=0.002) (Supplementary Figure 3). A similar trend was observed in the cumulative incidence of MACE and MALE (MACE, DES with HBR, 29.1%; DCB with HBR, 23.3%; DES with non-HBR, 7.6%; DCB with non-HBR, 9.1%; p=0.002; MALE, DES with HBR, 28.7%; DCB with HBR, 28.9%; DES with non-HBR, 15.6%; DCB with non-HBR, 9.6%; p=0.02, respectively) (Supplementary Figure 3).
Table 3. Clinical outcomes.
| Patients with events (Cumulative 5-year incidence) |
|||||
|---|---|---|---|---|---|
| Outcomes | HBR | Non-HBR | HR | 95% CI | p-value |
| Major bleeding (BARC 3 or 5) | 86 (31.9) | 3 (2.3) | 8.52 | 2.69-27.0 | <0.001 |
| Intracranial bleeding | 15 (5.7) | 1 (0) | 4.46 | 0.59-33.8 | 0.15 |
| Gastrointestinal bleeding | 26 (12.0) | 1 (1.4) | 7.32 | 0.99-54.0 | 0.051 |
| Access site bleeding | 8 (3.6) | 1 (1.0) | 2.18 | 0.27-17.5 | 0.46 |
| Any procedural bleeding | 12 (5.8) | 0 (0) | NA | NA | 0.06 |
| Transfusion | 14 (5.2) | 0 (0) | NA | NA | 0.051 |
| Other bleeding | 19 (7.8) | 0 (0) | NA | NA | 0.02 |
| BARC type 5 | 8 (2.3) | 0 (0) | NA | NA | 0.13 |
| MACE | 149 (49.4) | 14 (17.5) | 3.14 | 1.82-5.44 | <0.001 |
| Death | 134 (44.4) | 11 (12.2) | 3.61 | 1.95-6.68 | <0.001 |
| Cardiovascular | 46 (19.0) | 6 (8.5) | 2.25 | 0.96-5.27 | 0.06 |
| Non-cardiovascular | 87 (31.3) | 5 (4.1) | 5.17 | 2.10-12.7 | <0.001 |
| Myocardial infarction | 9 (2.4) | 2 (1.0) | 1.32 | 0.28-6.12 | 0.73 |
| Stroke | 22 (9.0) | 2 (5.7) | 3.09 | 0.72-13.2 | 0.13 |
| MALE | 115 (41.4) | 21 (32.9) | 1.68 | 1.05-2.67 | 0.03 |
| Acute thrombosis | 8 (3.5) | 2 (3.8) | 1.12 | 0.24-5.28 | 0.89 |
| Reintervention | 103 (39.5) | 19 (32.9) | 1.54 | 0.97-2.47 | 0.07 |
| Major amputation | 17 (6.6) | 0 (0.0) | NA | NA | 0.03 |
| Data are expressed as number (percentage). The number of patients with events was counted until the end of follow-up. Cumulative 5-year incidence was estimated by the Kaplan-Meier method. HR with 95% CI of the HBR group relative to the non-HBR group of the outcome measures were estimated throughout the entire follow-up period by the Cox proportional hazards models. BARC: Bleeding Academic Research Consortium; CI: confidence interval; HBR: high bleeding risk; HR: hazard ratio; MACE: major adverse cardiovascular events; MALE: major adverse limb events; NA: not applicable | |||||
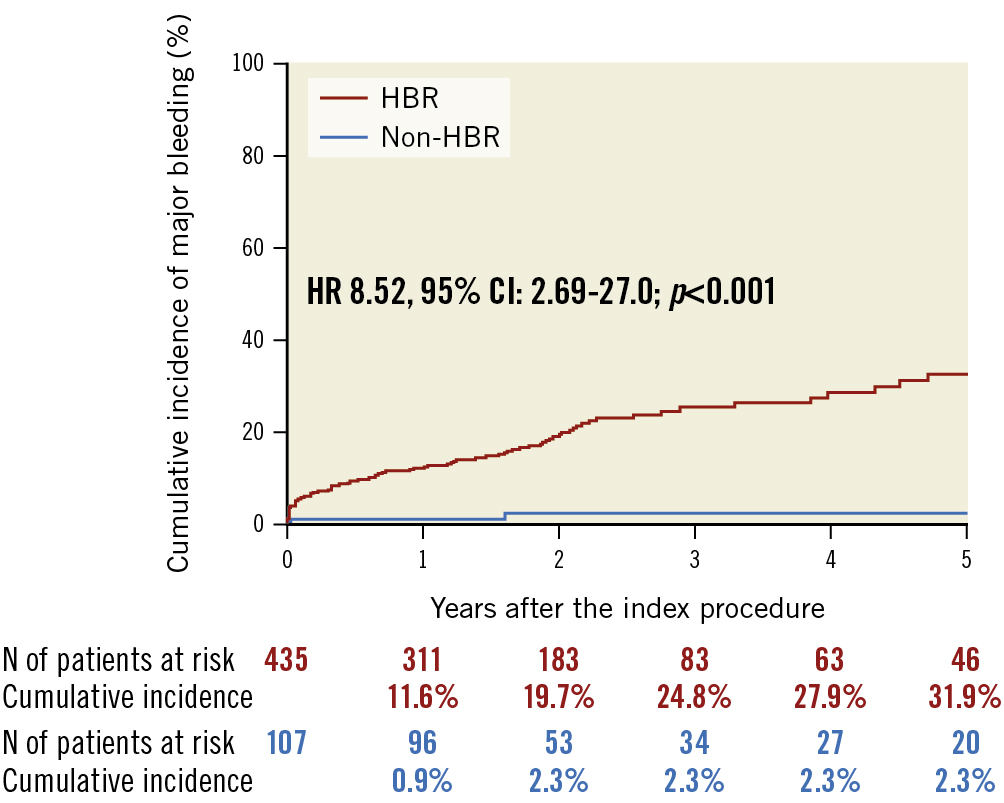
Figure 3. Major bleeding events in HBR and non-HBR patients. CI: confidence interval; HBR: high bleeding risk; HR: hazard ratio; N: number
Table 4. Mortality rates following major bleeding events.
| Outcome | Patient-y | Death | Mortality rate (Per 10 patient-y) |
Crude HR (95% CI) |
p-value | Adjusted HR (95% CI)* |
p-value |
|---|---|---|---|---|---|---|---|
| No/before major bleeding events | 1,238.1 | 93 | 0.75 | 1.00 | 1.00 | ||
| After major bleeding events | 92.6 | 48 | 5.19 | 6.82 (4.73-9.81) | <0.001 | 5.42 (2.91-10.1) | <0.001 |
| *Adjusted for age, body mass index (<22 kg/m2), current smoker, diabetes mellitus, dyslipidaemia, dual antiplatelet therapy, haemoglobin (11 g/dl or not), haemodialysis, hypertension, male sex, platelet (<10×104/μl), eGFR (<30 ml/min/1.73 m2 without haemodialysis), oral anticoagulant use, prior heart failure, prior myocardial infarction, prior stroke, prior percutaneous coronary intervention. Major bleeding events used as a time-dependent covariate. CI: confidence interval; eGFR: estimated glomerular filtration rate; HR: hazard ratio; patient-y: patient-years | |||||||
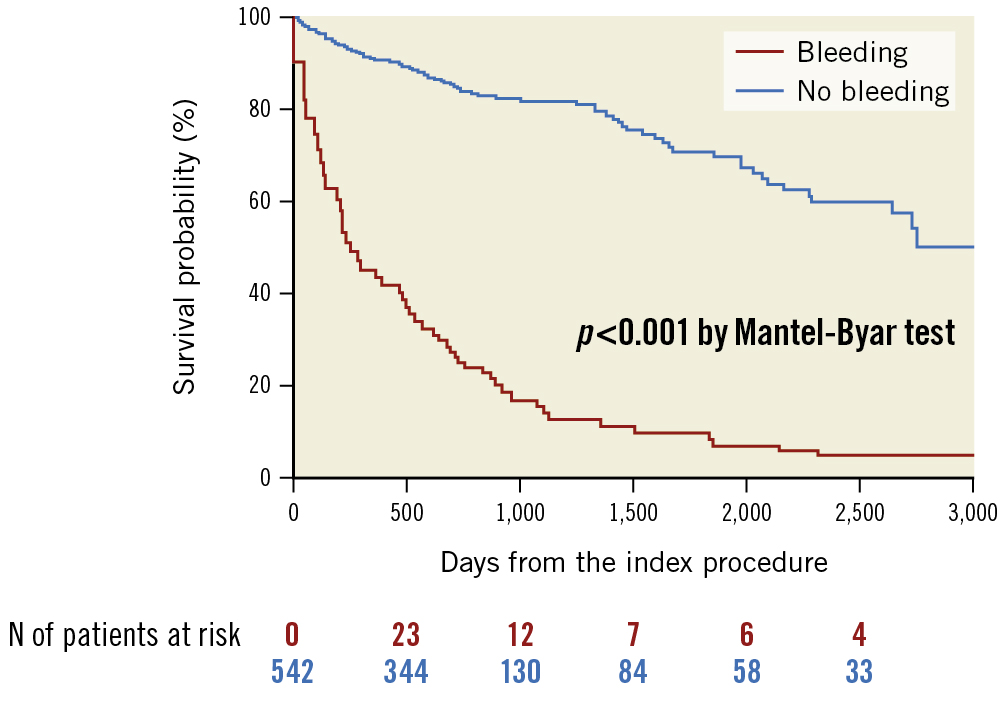
Figure 4. Survival probabilities before and after major bleeding events. N: number
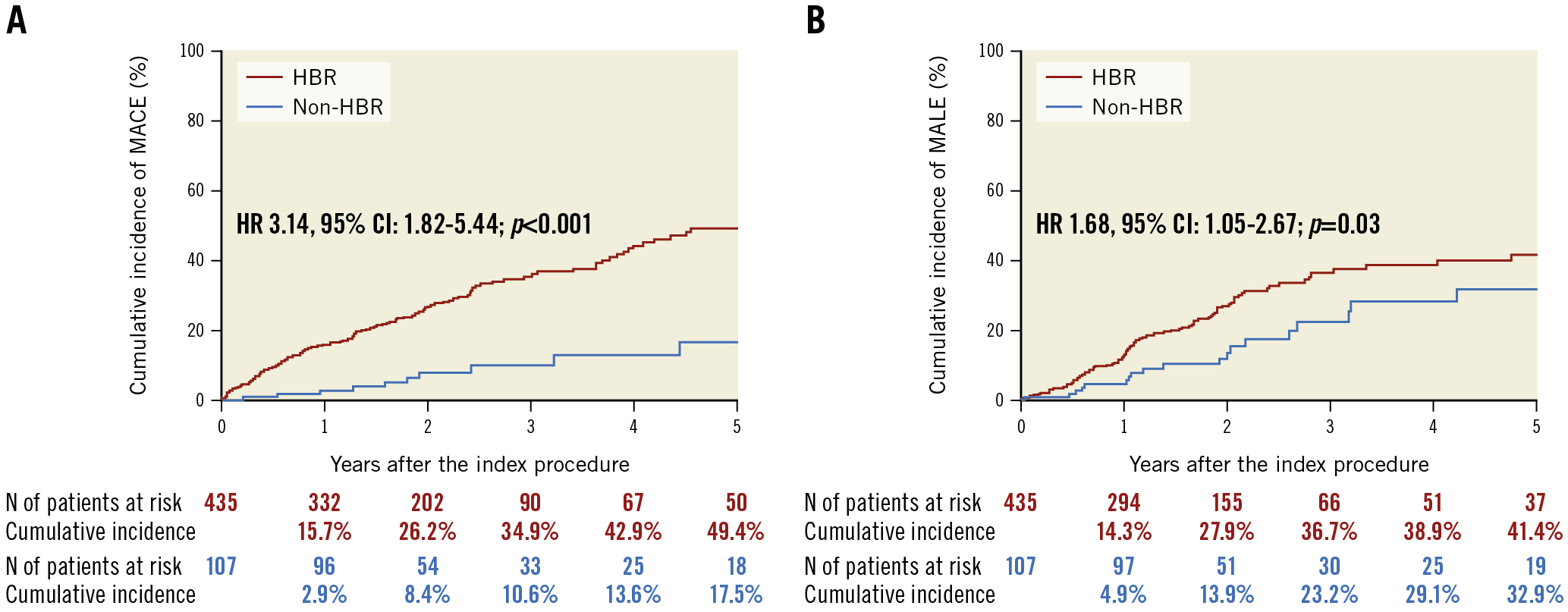
Figure 5. MACE and MALE in HBR and non-HBR patients. CI: confidence interval; HBR: high bleeding risk; HR: hazard ratio; MACE: major adverse cardiovascular events; MALE: major adverse limb events; MI: myocardial infarction; N: number
Effects of individual ARC-HBR criteria on major bleeding
Severe CKD, severe anaemia, oral anticoagulation, and spontaneous bleeding requiring hospitalisation or transfusion within the past 6 months, except for thrombocytopaenia, liver cirrhosis, active malignancy, previous intracranial haemorrhage (ICH), and major surgery on DAPT, were associated with a major bleeding risk ≥4% at 1 year for those who met major criteria. Furthermore, for patients aged ≥75 years, mild anaemia and any ischaemic stroke at any time not meeting the major criterion, moderate CKD, and long-term use of oral non-steroidal anti-inflammatory drugs and steroids, except for a bleeding event within 12 months, were associated with major bleeding risk ≥4% at 1 year for those meeting minor criteria (Supplementary Figure 4).
Effects of numbers of ARC-HBR major and minor criteria on major bleeding events
The cumulative 5-year incidence of major bleeding incrementally increased as the number of ARC-HBR major criteria increased (≥2 major: 48.6%, 1 major: 33.1%, ≥2 minor 12.9%, and non-HBR: 2.3%; p<0.001) (Supplementary Figure 5).
Predictors of major bleeding
The predictors of major bleeding were severe CKD (HR 2.36, 95% CI: 1.45-3.86; p=0.001), heart failure (HR 2.07, 95% CI: 1.25-3.41; p=0.007), and severe anaemia (HR 2.04, 95% CI: 1.27-3.28; p=0.003) (Table 5).
Table 5. Predictors of major bleeding events.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| ≥75 years | 1.53 | 0.99-2.38 | 0.054 | 1.41 | 0.91-2.19 | 0.12 |
| Male sex | 1.11 | 0.71-1.77 | 0.65 | 1.50 | 0.94-2.38 | 0.09 |
| Oral anticoagulant | 1.29 | 0.73-2.17 | 0.37 | 1.19 | 0.68-2.08 | 0.53 |
| Severe CKD | 3.39 | 2.20-5.33 | <0.001 | 2.36 | 1.45-3.86 | 0.001 |
| Severe anaemia | 3.13 | 2.05-4.78 | <0.001 | 2.04 | 1.27-3.28 | 0.003 |
| Bleeding within 6 months | 2.42 | 0.59-6.46 | 0.19 | 2.02 | 0.62-6.52 | 0.29 |
| Heart failure | 2.51 | 1.53-4.00 | 0.001 | 2.07 | 1.25-3.41 | 0.007 |
| CLTI | 2.21 | 1.43-3.38 | <0.001 | 1.38 | 0.87-2.18 | 0.17 |
| Severe CKD defined as estimated glomerular filtration rate (eGFR) <30 L/min/1.73 m2. Severe anaemia defined as haemoglobin level <11 g/dL. CI: confidence interval; CKD: chronic kidney disease; CLTI: chronic limb-threatening ischaemia; HR: hazard ratio | ||||||
Discussion
The main findings of this study were as follows: (1) ARC-HBR was identified in 80.3% of patients undergoing EVT for femoropopliteal artery lesions treated with DCD; (2) the cumulative 5-year incidence of major bleeding events incrementally increased as the number of ARC-HBR major criteria increased; (3) HBR patients showed a higher incidence of MACE and MALE than those without HBR; (4) severe CKD, heart failure, and severe anaemia were independent predictors of major bleeding events; and (5) major bleeding events were associated with a 5.4-fold increased risk of mortality after EVT.
PAD is a relatively common disease in an ageing society, with 220 million affected globally and an increasing prevalence worldwide13. Given that the evolution of EVT over the last 20 years has facilitated the treatment of an increasingly complex patient population, the identification and management of HBR in patients undergoing EVT is becoming as crucial as for those undergoing PCI. Previous studies reported that the ARC-HBR was identified in ~40% to 50% of CAD patients undergoing PCI23. Since patients with PAD have a higher risk profile than those with CAD6, they may be at HBR more frequently than CAD patients. Indeed, PAD has reportedly been associated with bleeding events in the Japanese population and is included as a major criterion in the Japanese version of the ARC-HBR criteria14. Recent studies reported that 69.7% to 75.0% of patients undergoing EVT met the ARC-HBR criteria, suggesting a higher prevalence of HBR in patients undergoing EVT than previously reported in those undergoing PCI231516. However, these results were hampered by a relatively small number of patients, varying target arteries, and a low frequency of contemporary DCD use. The present study showed that the ARC-HBR criteria were met in 80.3% of PAD patients undergoing EVT for femoropopliteal artery lesions with DCD, which was higher than in previous studies1516. A possible explanation for this is that the present study had a considerably complex patient population (e.g., advanced age, a high prevalence of CKD and anaemia), reflecting contemporary EVT practice. Accordingly, physicians should recognise that HBR is common in PAD patients undergoing EVT in clinical practice.
Recent studies have verified the applicability of the ARC-HBR criteria in all-comer registries, demonstrating that a major bleeding risk of ≥4% at 1 year appeared acceptable in HBR patients in a real-world setting23. However, whether the ARC-HBR criteria can be applied to PAD patients undergoing EVT remains undetermined. In the present study, the cumulative incidence of BARC 3 or 5 bleeding was 11.6% and 31.9% at 1 and 5 years, respectively; only 2.3% of non-HBR patients experienced major bleeding events during the follow-up, which was in line with previous studies216. Notably, the sensitivity and specificity of the ARC-HBR versus non-ARC-HBR for 5-year major bleeding events were 0.98 and 0.26, respectively. Furthermore, the ARC-HBR criteria had good discriminating power for 5-year major bleeding events at the cut-off of ≥2 points (1 major or ≥2 major) vs <2 points (≥2 minor or non-HBR). These findings suggested that the bleeding risk of patients undergoing EVT was well differentiated using the ARC-HBR criteria. In contrast, the 1-year incidence rate of BARC 3 or 5 bleeding in HBR patients undergoing EVT varied widely across the studies, ranging from 5.7% to 12.6%1516. This might be explained by differences in patient background, EVT procedures, and antithrombotic regimens. In particular, a transradial approach could potentially reduce access-site bleeding among the major bleeding events17. Even though several limitations in the technical aspect still exist, transradial EVT should be considered in HBR patients to prevent procedural bleeding events.
Patients with HBR present a higher risk of adverse outcomes after PCI, attributed to advanced age and more significant comorbidities23. Recently, some studies showed midterm outcomes after EVT in HBR patients compared with non-HBR patients1516. Yoshioka et al reported that all-cause death and bleeding events occurred in only HBR patients at 1 year (14.7% and 16.9%, respectively)15. Similarly, Hashimoto et al reported that only HBR patients (7.8%) died during 1-year follow-up16. These results suggested that the ARC-HBR criteria would be informative for the risk stratification of midterm outcomes in PAD patients undergoing EVT. To date, however, it remains unknown whether the ARC-HBR criteria could apply to the risk stratification of long-term outcomes (beyond 1 year) after EVT. The present study demonstrated that the cumulative 5-year incidence of MACE and MALE were significantly higher in HBR patients than non-HBR patients. Intriguingly, a 1-year landmark analysis demonstrated that MACE in HBR patients continued to occur without any attenuation, while MALE did not differ between HBR and non-HBR patients. The possible explanations for this included the following: (1) the 5-year incidence of major amputation was relatively low, and (2) reintervention occurred mainly within 1 year. Thus, in terms of mid- and long-term outcomes, the ARC-HBR criteria might help identify higher-risk PAD patients at the index procedure.
Current guidelines recommend at least 1-month DAPT after EVT, whereas no consensus exists on the optimal DAPT duration1819. A meta-analysis demonstrated that DAPT reduced MACE and MALE after EVT in PAD patients compared with aspirin alone, although the DAPT duration was heterogeneous among the included studies20. Recently, Cho et al reported that DAPT ≥6 months reduced 5-year MACE and MALE without increasing bleeding events21. Given these findings, prolonged DAPT might improve the outcomes of PAD patients undergoing EVT. On the contrary, the current study demonstrated that HBR patients had worse outcomes with a higher incidence of major bleeding events over the 5-year follow-up. Of note, increasing numbers of the ARC-HBR criteria were associated with an incrementally higher incidence of major bleeding events, in line with previous PCI studies23. A longer DAPT duration after PCI is generally associated with a lower rate of thrombotic events, but it carries a higher bleeding risk that may adversely affect mortality22. Until now, however, it has remained unclear whether major bleeding events were associated with mortality after EVT. To our knowledge, the current study was the first to demonstrate that major bleeding events contribute independently to mortality after EVT. Accordingly, preventing major bleeding events may assist in the improvement of outcomes in PAD patients undergoing EVT.
Limitations
The current study has several limitations. First, since this was a retrospective, observational, single-centre study, selection bias might have affected the conclusion. Nevertheless, the study enabled sufficient data collection and complete validation of the ARC-HBR criteria. Second, the bleeding events were not independently adjudicated by a blinded clinical events committee, which might have resulted in potential reporting bias. Third, we could not collect frailty status (i.e., clinical frailty score), cognitive function, and unpredicted confounders in the present study. Fourth, because of the lack of medical compliance over the follow-up, the association between antiplatelet therapy and bleeding events was inconclusive in the present study. Further studies are warranted to establish the optimal antiplatelet therapy regimen based on individual bleeding and thrombotic risk in PAD patients, especially those with HBR. Finally, extrapolation of our results outside Japan requires caution because the study population consisted solely of Japanese subjects.
Conclusions
The ARC-HBR criteria were met by 80.3% of PAD patients undergoing EVT for femoropopliteal artery lesions with contemporary DCD. Compared with non-HBR patients, those with HBR had an excess risk of major bleeding events, MACE, and MALE during the long-term follow-up. Given that major bleeding events remarkably increased the risk of mortality after EVT, the ARC-HBR criteria might be helpful for the risk stratification of PAD patients who undergo EVT with contemporary DCD.
Impact on daily practice
The ARC-HBR criteria were met by ~80% of PAD patients undergoing EVT with contemporary DCD. Patients with HBR had an excess risk of major bleeding events, MACE, and MALE after EVT procedures, and the ARC-HBR criteria might be helpful for the risk stratification of these patients. Further studies are warranted to establish the optimal antiplatelet therapy regimen based on individual bleeding and thrombotic risk in patients with PAD, especially those with HBR.
Acknowledgements
The authors thank the catheterisation laboratory staff for their assistance with this work.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

