
Abstract
Aims: This study sought to investigate differences in vascular response between self-expanding bare metal nitinol stents (BMS) and paclitaxel-eluting nitinol stents (PES), in superficial femoral artery (SFA) disease, using optical frequency domain imaging (OFDI).
Methods and results: Six months after stent implantation, follow-up quantitative vascular angiography (QVA) and OFDI assessment were scheduled to evaluate vascular response. Volume index (VI) was defined as volume divided by stent length. The primary endpoint was OFDI-derived late lumen area loss, defined as lumen VI post stent implantation minus lumen VI at follow-up. A total of 28 SFA lesions were analysed, with cases randomised to receive either BMS or PES implantation. QVA-derived diameter stenosis at six-month follow-up was lower in the PES group than in the BMS group (28.5% vs. 39.7%, p=0.04). After six months, BMS VI increased by 33.8% (20.7±3.7 to 27.7±3.5 mm3/mm), whilst PES exhibited an increase of 32.1% (19.0±2.3 to 25.1±4.7 mm3/mm). Neointimal VI was smaller (7.4±2.6 mm3/mm vs. 10.5±3.2 mm3/mm, p<0.01) and late lumen area loss was lower (2.9±1.3 mm3/mm vs. 5.6±2.8 mm3/mm, p<0.01) in the PES group.
Conclusions: Serial volumetric OFDI analyses confirmed significantly smaller amounts of neointimal tissue and lower late lumen area loss following PES implantation for SFA lesions at short-term follow-up.
Abbreviations
BMS: bare metal stent
DES: drug-eluting stent
EVT: endovascular therapy
FP: femoropopliteal
ISR: in-stent restenosis
OCT: optical coherence tomography
OFDI: optical frequency domain imaging
PES: paclitaxel-eluting stent
QVA: quantitative vascular angiography
SFA: superficial femoral artery
VI: volume index
Introduction
In femoropopliteal (FP) artery lesions, endovascular therapy (EVT) with self-expanding bare metal nitinol stents (BMS) has shown superior outcomes when compared with balloon angioplasty1. A previous study has reported a primary patency rate of approximately 80% (at one year) following BMS implantation in superficial femoral artery (SFA) lesions2. However, BMS implantation in FP lesions remains limited by the phenomenon of in-stent restenosis (ISR), due to neointimal hyperplasia. Similar to those used in the treatment of coronary artery lesions, drug-eluting stents (DES) have shown better clinical outcomes than BMS in the periphery3. The primary patency rate following implantation of self-expanding, paclitaxel-eluting nitinol stents (PES) in SFA lesions was reported to be 90% at one year3. Nevertheless, the mechanism of vascular response underlying the superior patency of PES remains obscure.
Intravascular optical coherence tomography (OCT) is a light-based imaging modality, providing high-resolution images of the coronary artery4. Recently, a second-generation OCT technique, optical frequency domain imaging (OFDI), has been developed to provide higher imaging resolutions and greater depth penetration than conventional time-domain OCT5. OFDI allows intravascular imaging with a greater scan depth and faster pullback speed, which appears to be suitable for the evaluation of longer lesions with larger vessel diameters, such as FP lesions6. This study was designed to investigate the difference in vascular response between BMS and PES implantation in SFA lesions, using serial OFDI analysis.
Methods
STUDY DESIGN AND PATIENTS
We conducted a sub-analysis as part of a prospective, randomised study comparing strut coverage between BMS and PES implantation at six months post treatment, in a consecutive series of patients with SFA disease6. Among 40 randomised de novo SFA lesions, 30 lesions (15 lesions treated with BMS and 15 lesions with PES) were pre-specified as the serial OFDI sub-analysis group at enrolment, and were scheduled to receive OFDI examinations both post stent implantation and at a six-month follow-up. Patients with symptomatic peripheral artery disease resulting from de novo SFA lesions were assessed as eligible for an OFDI examination (lesion length ≤15 cm and vessel diameter <8 mm), and were subsequently enrolled. Exclusion criteria were: 1) acute limb ischaemia, 2) chronic kidney disease, and 3) previous bypass surgery or previous EVT in the SFA. The protocol was approved by the institutional review board of Hyogo College of Medicine, with written, informed consent obtained from all patients before participation.
EVT PROCEDURE
Primary stent implantation for SFA lesions was performed via the contralateral femoral approach. Predilation was performed using a balloon with a diameter equal to that of the distal reference vessel, according to angiographic visual estimation. Following predilation, BMS (S.M.A.R.T.® Control™; Cordis, Johnson & Johnson, Miami Lakes, FL, USA) or PES (Zilver® PTX®; Cook Medical, Bloomington, IN, USA) implantations were performed. Stents with a diameter 1-2 mm larger than vessel diameters at the reference sites of target lesions were chosen. Post-dilation was performed routinely in all lesions, using a balloon with a 5 mm diameter at a pressure of 14 atmospheres. Aspirin 100 mg/day and clopidogrel 75 mg/day were started at least one week prior to EVT, and were continued for at least six months following EVT.
QUANTITATIVE VASCULAR ANGIOGRAPHY ANALYSIS
Angiographies were obtained pre-EVT, post stent implantation, and at the six-month follow-up. Quantitative vascular angiography (QVA) analysis was performed using commercially available software (CAAS 5.9; Pie Medical Imaging, Maastricht, The Netherlands), in a blinded fashion. The tip of a 6 Fr guiding catheter was used for calibration in the QVA analysis. Any zooming or panning was not allowed during angiography. Minimum lumen diameter (MLD), reference vessel diameter, and total stent length were obtained. Diameter stenosis was calculated as follows: MLD/average ([proximal+distal]×½) reference vessel diameter7.
OFDI ACQUISITION
OFDI examination was performed with a Terumo OFDI system (FastView®; Terumo Corp., Tokyo, Japan), using a non-occlusive technique. A 6 Fr guiding catheter was advanced past the common femoral artery and positioned 10-20 mm proximal to the stent edge. The OFDI catheter was advanced over a 0.014 inch guidewire, with the imaging core placed distally to the stent. Automated OFDI pullback with a speed of 40 mm/s was performed with continuous injection of 50% contrast medium, at a flow rate of 8 mL/s, from the guiding catheter.
OFDI ANALYSIS
Collected OFDI data were analysed in an independent core laboratory at Stanford University Medical Center (Cardiovascular Core Analysis Laboratory), which was blinded to the clinical information. OFDI analysis was performed with PC-based software (echoPlaque™; Indec Systems Inc, Santa Clara, CA, USA). Image slices were reviewed at every 5 mm axial interval throughout the complete stented segment. All cross-sectional imaging slices were initially screened for quality assessment and excluded from analysis if any portion of the stent was out of the screen (framing out) or if the image had insufficient image quality caused by residual blood, artefact, or reverberation. Lumen and stent area were manually traced, and the neointimal area (stent area minus lumen area) was calculated. Cross-sectional narrowing (CSN) was defined as neointimal area divided by stent area. Neointimal volume was calculated as stent volume minus lumen volume, and neointimal volume obstruction was expressed as neointimal volume divided by stent volume. Each volume was divided by stent length to adjust for difference in stent length (volume index [VI], mm3/mm). Late lumen area loss was defined as lumen VI post stent implantation, minus that measured at the six-month follow-up. To evaluate the axial distribution of neointimal tissue through the stented segment, each stent was divided into eight segments along the longitudinal axis, with average neointimal VI of each segment then summarised. The presence or absence of peri-strut low intensity area (PLIA) was evaluated at the imaging slice with minimum lumen area (MLA)8. Intraluminal material, defined as any mass protruding into the luminal surface within the stented segment, regardless of size or extent of signal attenuation, was also recorded. Stent edge dissection was defined as a disruption of the vessel luminal surface at the stent edges with a visible flap.
At strut level analysis, each stent strut was classified into one of three categories: (i) well-apposed to the vessel wall with apparent tissue coverage (protruding/covered strut), (ii) well-apposed to the vessel wall without tissue coverage (well-apposed and uncovered strut), and (iii) malapposed to the vessel wall without tissue coverage (malapposed and uncovered strut)9. If neointimal coverage on the strut was observed at follow-up, neointimal tissue thickness, defined by the distance between the luminal surface of the covering tissue and the luminal surface of the strut, was measured.
To assess the reliability of quantitative OFDI measurements, intra- and inter-observer variability analyses were performed on measurements of lumen area and stent area, on a total of 50 randomly selected cross-sections from 10 lesions. The images were re-evaluated by a single observer to identify intra-observer variability, at two separate time points. The inter-observer variability was ascertained from measurements conducted by two independent observers.
ENDPOINTS AND STATISTICAL ANALYSIS
The primary endpoint of this study was late lumen area loss at six-month follow-up. Categorical variables were compared using the chi-squared test or Fisher’s exact test in the case of low counts. Continuous variables are expressed as mean±standard deviation or median with interquartile range. The normality of the continuous variables was confirmed with the Shapiro-Wilk test. For continuous variables, comparisons between BMS and PES were performed with an unpaired t-test or the Mann-Whitney U test, and comparisons between post stent implantation and follow-up via a paired t-test or Wilcoxon signed-rank test. For strut level OFDI measurements, multilevel analysis was performed in order to take account of clustering of the values within each subject10. The reliability of continuous variables in OFDI measurements was expressed as the intra-class correlation coefficient. To assess the influence of chronic vessel injury from self-expanding stents on neointimal proliferation or stent patency, Pearson’s correlation analysis was performed to examine the relation between increases in stent VI over six months and neointimal VI, or lumen VI, at follow-up. A p-value of <0.05 was considered statistically significant.
Results
PATIENTS, LESIONS, AND PROCEDURES
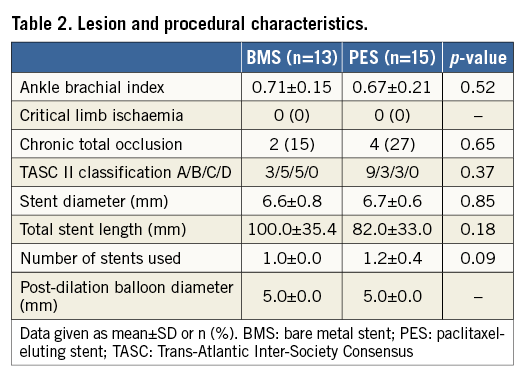
Among 30 de novo SFA lesions from 28 patients pre-specified as the serial OFDI sub-analysis group, one lesion which was treated with BMS required target lesion revascularisation due to recurrence of a symptom before the follow-up. Additionally, one patient with BMS refused the follow-up examination. Finally, 28 lesions (13 lesions; BMS group, and 15 lesions; PES group) from 26 patients, with complete serial OFDI images post stent implantation and at the six-month follow-up, were analysed. Of these, two patients had bilateral SFA diseases in both lower limbs; there was no case which had multiple lesions in the same SFA. Patient baseline clinical characteristics were well matched between both groups (Table 1). Lesion and procedural data are shown in Table 2. Total stent length was 100.0±35.4 mm in the BMS group and 82.0±33.0 mm in the PES group (p=0.18).
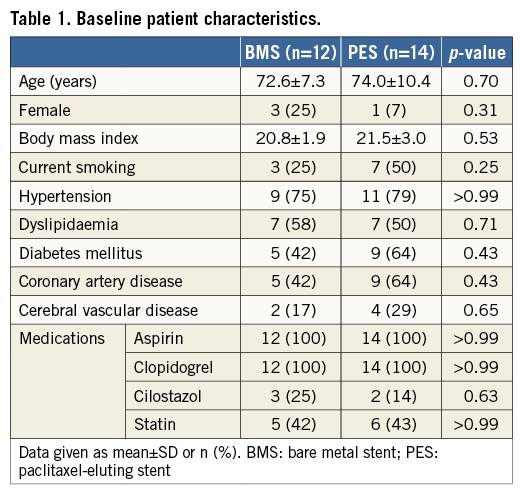
QUANTITATIVE VASCULAR ANGIOGRAPHY FINDINGS
QVA measurements are listed in Table 3. There were no differences in MLD of the target lesion pre-EVT or post stent implantation between the two groups in QVA analysis. Percent diameter stenosis at the six-month follow-up was significantly lower in the PES group than in the BMS group (28.5% vs. 39.7%, p=0.04). Angiographic late lumen diameter loss was smaller in the PES group (0.83±0.55 mm vs. 1.40±0.80 mm, p=0.03).
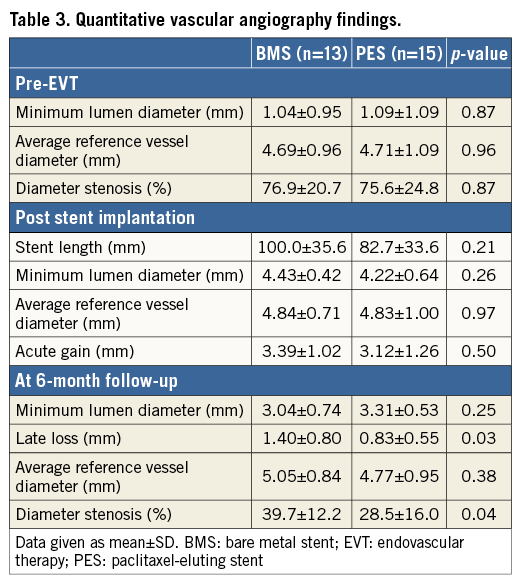
SERIAL OFDI FINDINGS
A total of 1,111 imaging slices were initially reviewed; 16 (1.4%) slices were excluded from analysis due to framing out and 39 (3.5%) slices due to residual blood. OFDI measurements of lumen area exhibited excellent intra- and inter-observer reliability. Intra-class correlation coefficients for intra-observer and inter-observer variability of lumen area were 0.999 and 0.999, respectively. Stent area also showed excellent intra- and inter-observer reliability (0.995 and 0.995). Serial OFDI findings are presented in Table 4. BMS increased stent VI 33.8% during the follow-up period, with PES increasing stent VI by 32.1% (Figure 1). The degree of increase in stent VI was similar between the two groups (p=0.33). Neointimal VI was 10.5±3.2 mm3/mm in the BMS group and 7.4±2.6 mm3/mm in the PES group (p<0.01). Lumen VI decreased during follow-up in both the BMS (from 22.9±3.9 to 17.2±4.8 mm3/mm, p<0.001) and PES groups (from 20.8±2.2 to 17.9±3.0 mm3/mm, p<0.001). Intraluminal material was detected in nine lesions post stent implantation, with most disappearing by follow-up. A total of nine non-flow-limiting edge dissections (four in the BMS group and five in the PES group) were identified on OFDI post stent implantation. All of those dissections were completely healed on follow-up OFDI, without thrombus formation or lumen loss. Changes in stent, neointimal, and lumen VI during the follow-up period are summarised in Figure 2. In the PES group, late lumen area loss was smaller than that in the BMS group (2.9±1.3 mm3/mm vs. 5.6±2.8 mm3/mm, p<0.01), due to a smaller degree of neointima.
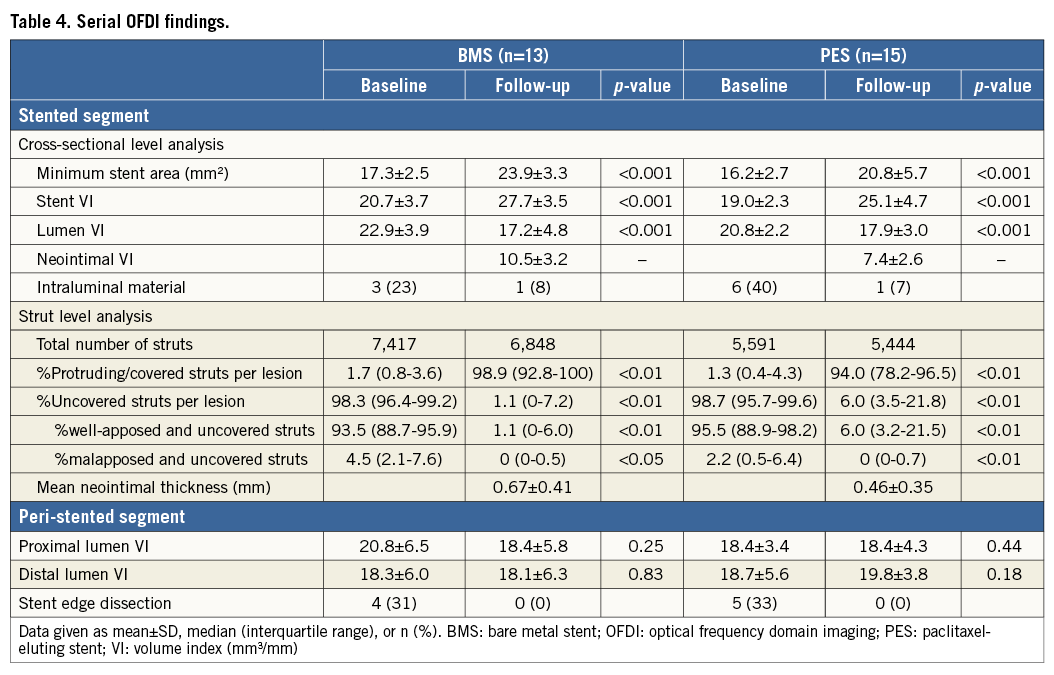
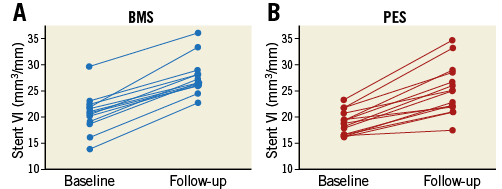
Figure 1. Increases in stent VI during the follow-up period. A) BMS group. B) PES group. BMS increased 33.8% in stent VI, and PES increased 32.1% in stent VI.
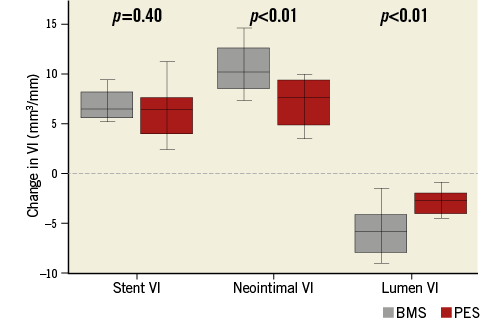
Figure 2. Changes in stent, neointimal, and lumen VI during the follow-up period. In the PES group, neointimal proliferation was significantly suppressed as compared to the BMS group. As a result, late lumen area loss (decrease in lumen VI) was smaller in the PES group.
A total of 14,265 baseline struts and 11,035 follow-up struts were reviewed at strut level analysis (Table 4). At baseline, the percentage of protruding struts per lesion was 1.7% (0.8-3.6) in the BMS group and 1.3% (0.4-4.3) in the PES group, whilst the observed percentage of malapposed and uncovered struts was 4.5% (2.1-7.6) in the BMS group and 2.2% (0.5-6.4) in the PES group. At the six-month follow-up, malapposed struts had almost disappeared in both groups.
NEOINTIMAL PROLIFERATION
The percentage of neointimal volume obstruction was smaller (28.7±7.4% vs. 38.6±13.0%, p=0.02) and maximum CSN lower (52.6±5.9% vs. 61.4±14.4%, p=0.04) in the PES group than those in the BMS group (Table 5). The presence of PLIA was 69.2% in the BMS group and 61.5% in the PES group (p=0.46). Axial distributions of neointimal VI throughout the stented segment are shown in Figure 3. In the BMS group, neointimal tissue was distributed evenly along the longitudinal axis throughout the entire stented segment. In contrast, greater quantities of neointimal tissue were observed at the distal portion of the stented segment when compared to the proximal portion, in the PES group. Figure 4 shows the association between chronic stent enlargements during the follow-up period and degrees of neointimal proliferation or lumen area at the follow-up. Pearson’s correlation analyses produced excellent correlations between neointimal VI and an increase in stent VI in both the BMS (r=0.756, p<0.01) (Figure 4A) and PES groups (r=0.937, p<0.001) (Figure 4B). In the BMS group, there was no correlation between lumen VI and an increase in stent VI (r=–0.074, p=0.82) (Figure 4C). In the PES group, lumen VI at the follow-up directly correlated with an increase in stent VI (r=0.591, p=0.02) (Figure 4D).
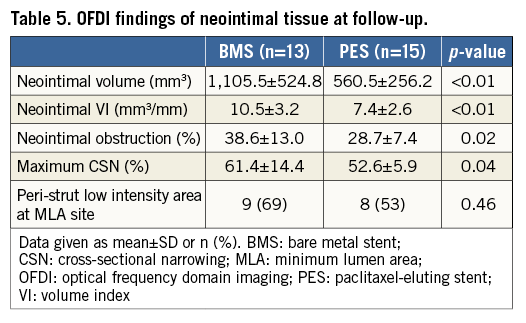
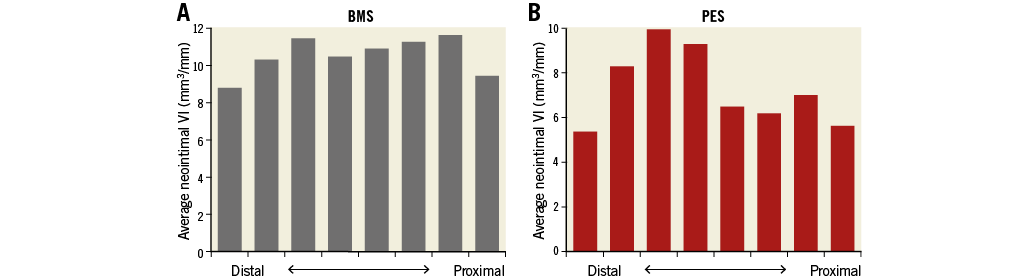
Figure 3. Axial distributions of neointimal tissue throughout the stented segment. A) BMS group. B) PES group. Neointimal VI was distributed evenly along the longitudinal axis throughout the entire stented segment in the BMS group. In comparison, larger quantities of neointimal VI were observed at the distal portion of the stented segment when compared to the proximal portion, in the PES group.
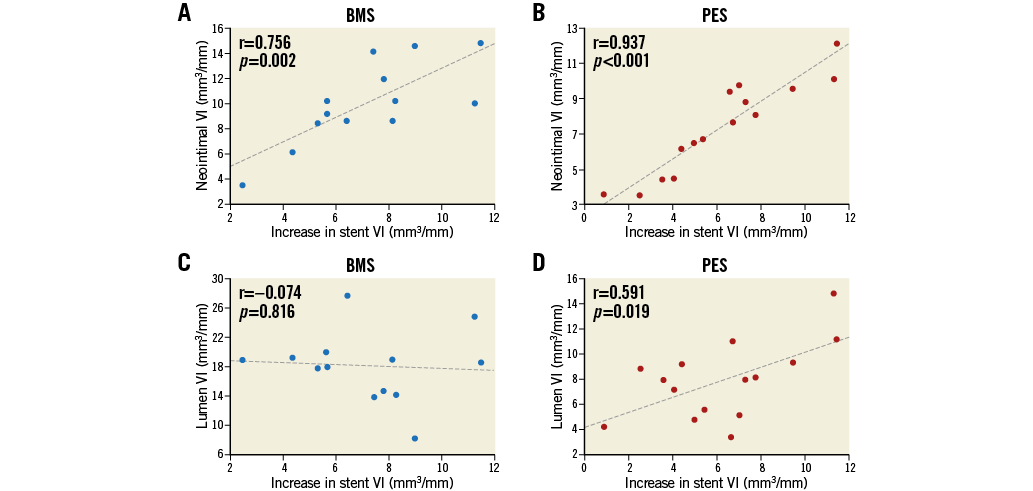
Figure 4. Correlations between an increase in stent VI, and neointimal VI (A, B) and lumen VI (C, D) at the follow-up. Neointimal VI was directly correlated with an increase in stent VI following BMS and PES implantation. Lumen VI at the follow-up also correlated with an increase in stent VI following PES implantation.
Discussion
The main findings of this study are as follows: 1) significantly smaller quantities of neointimal tissue resulted in lower late area loss following PES implantation, 2) abnormal OFDI findings (intraluminal materials, non-flow-limiting edge dissections, and malapposed struts), identified post stent implantation, were almost resolved at the chronic phase, and 3) the degree of neointimal proliferation was directly correlated with chronic enlargement of self-expanding stents. Although clinical trials have shown superior patency following PES implantation in FP lesions compared to BMS implantation3, data explaining the mechanism of superior patency in peripheral PES implantation are limited. To the best of our knowledge, this is the first study to report on the difference in chronic vascular response between BMS and PES implantation in FP lesions using serial volumetric OFDI analysis.
Incomplete stent apposition has been reported as a risk for late stent thrombosis in the coronary artery lesion11. In this study, malapposed struts recorded post stent implantation were significantly mitigated and almost resolved at the chronic phase in both groups. Additionally, amongst nine tissue protrusions (plaque or thrombus) detected after stenting, only two could be observed at follow-up. A previous OCT study showed that stent edge dissections without flow limitation in the coronary artery had a benign course and were not associated with an increased incidence of adverse outcomes12. A serial OCT analysis has also reported that non-flow-limiting edge dissections, detected by OCT only, resolved completely at the chronic phase13. In this study, OFDI detected nine, non-flow-limiting edge dissections post stent implantation, with none of these detected by angiography. Similar to the coronary artery lesion, edge dissections in SFA lesions were completely healed at follow-up, without restenoses or thrombus formation. These results suggest that incomplete stent apposition and non-flow-limiting edge dissections in the SFA, identified by OFDI, may have no impact on clinical outcomes.
Previous studies have reported that ISR is defined mechanistically by neointimal hyperplasia, which is an exaggerated healing response to vessel wall injury, occurring as a result of mechanical dilatation14,15. Kobayashi et al16 reported that self-expanding BMS deployed in the coronary artery increased stent volume 23.6% during a six-month follow-up period. In our study, self-expanding BMS and PES also increased stent volume (33.8% and 32.1%, respectively). This suggests that chronic, continuous mechanical injury from self-expanding stents on vessel walls may influence neointimal proliferation in the SFA. In this study, neointimal VI was directly correlated with an increase in stent VI, during the six months following BMS or PES implantation. However, there was no relation between neointimal VI and stent VI post stent implantation in the BMS group (r=-0.249, p=0.422) or the PES group (r=0.414, p=0.127). These results suggest that neointimal proliferation may be accelerated by continuous stent expansion force on vessel walls during the follow-up period, rather than the mechanical dilatation of the initial EVT procedure. Additionally, lumen VI at the follow-up, in the PES group, was directly correlated with an increase in stent VI. In lesions treated with self-expanding PES, lumen area at the chronic phase could be compensated with stent enlargement despite accelerated neointimal proliferation. In contrast, no association between lumen VI at follow-up and an increase in stent VI was observed in the BMS group, suggesting that neointimal proliferation, accelerated by the expansion force of BMS implantation, might induce larger lumen area loss. The precise reason for this discrepancy between BMS and PES remains unclear; however, we suggest that neointimal proliferation may be suppressed by paclitaxel, regardless of the degree to which the stent exerts continuous expansion force. This indicates that chronic mechanical injury from self-expanding stents may be involved in ISR, especially following self-expanding BMS implantation.
Study limitations
This study was a retrospective sub-analysis from a prospective randomised study6, with a relatively small study population. In the preceding randomised study, sample size calculation was performed according to the expected rate of uncovered stent struts, as observed in the HORIZONS-AMI trial17, with a minimum of 20 lesions in each group deemed necessary to demonstrate the difference of strut coverage between BMS and PES. Further prospective studies with larger numbers of patients are required to confirm our results. OFDI measurements prior to stent implantation (after guidewire passage through the target lesion) were not evaluated because an adequate imaging quality could not be obtained, due to residual blood prior to stenting. This may be a limitation of OFDI evaluation in FP lesions. Follow-up OFDI was conducted six months after stent implantation. However, further longer follow-up periods may be necessary to reach a conclusion on the vascular response for stent implantation in the SFA.
Conclusion
A serial volumetric OFDI analysis confirmed significantly smaller quantities of neointimal tissue and decreased late area loss following PES implantation for SFA lesions at short-term follow-up. PES may reduce restenosis by suppression of neointimal proliferation.
| Impact on daily practice Our data suggest that PES in the SFA may improve patency by suppression of neointimal proliferation without an increase of abnormal neointimal findings compared with BMS. No adjunctive intervention might be required for abnormal findings (intraluminal materials, non-flow-limiting edge dissections, and malapposed struts) which were identified on OFDI post stent implantation. Excess chronic stent expansion force on vessel walls might be associated with ISR, especially following self-expanding BMS implantation. |
Acknowledgements
The authors thank the staff in the catheterisation laboratory at Hyogo College of Medicine for their excellent assistance.
Conflict of interest statement
The authors have no conflicts of interest to declare.

