Abstract
Aims: The purpose of this prospective clinical investigation was to quantify the degree and range of compressive and bending deformations sustained by self-expanding nitinol stents when implanted into the femoropopliteal arteries of patients with symptomatic peripheral vascular disease (PVD).
Methods and results: Twenty-three nitinol self-expanding stents (Absolute®; Abbott Vascular, Santa Clara, CA, USA) with diameters ranging from 5-10 mm and lengths ranging from 40-100 mm were implanted in 19 lesions in 18 extremities of 17 patients. Two days following implantation, in vivo stent compression and bending were assessed by measurement of stent length and deflection angle via lateral view radiographs. The results showed that leg flexion was associated with significant stent bending; popliteal stents bent almost 90° while SFA stents bent only minimally. Leg flexion was also associated with stent shortening (compression), the greatest amount being observed for stents implanted into the popliteal artery (popliteal 8.5%±3.2%; SFA/prox pop 5.3%±0.5%; SFA 3.1%±1.8%). After a mean follow-up of 7.1±1.3 months, the degree of stent deformation during leg flexion was essentially unchanged as compared to immediate post-procedure levels.
Conclusions: Indwelling nitinol stents are routinely bent and compressed during leg flexion, with most bending observed in stents implanted near the popliteal artery. The degree of deformation observed immediately after implantation appears to be predictive of the chronic state, as repeat measurements obtained after a mean of seven months were essentially unchanged from baseline.
Introduction
Intravascular stents have become useful adjuncts to percutaneous revascularisation. Stenting is now routinely applied following balloon dilatation within coronary, carotid, renal and peripheral arteries. However, increasing clinical experience has led to a heightened awareness of the potential for stent fracture following implantation. While only occasionally observed in the coronary1,2, carotid3, renal4,5 and iliac arteries6, stent fracture after implantation into the femoropopliteal arteries is alarmingly common, as high as 65% in one clinical report7.
The mechanisms behind the high rate of strut fracture in stented femoropopliteal arteries have not been fully elucidated. One attractive hypothesis is that fracture may be a function of the unique biomechanical forces exerted on stents dwelling in the SFA8. Movement of the legs is a complex motion, as loading of the hips and knees during ambulation repeatedly compresses the arteries axially and can even produce multidimensional bends, twists and kinks. Several groups have attempted to measure and model these forces in vitro and in vivo8-10. To date, however, these studies have been limited to either unstented arteries or stented arteries within cadaveric specimens. No studies are yet available which document the character and degree of deformations in actual stented arteries in intact patients and how deformations might change over time. The purpose of this prospective clinical investigation was to quantify the degree and range of compressive and bending deformations encountered by self-expanding nitinol stents after implantation into patients with peripheral vascular disease (PVD).
Methods
Seventeen patients with symptomatic PVD who underwent percutaneous transluminal angioplasty and primary stenting (Absolute®; Abbott Vascular, Santa Clara, CA, USA) of femoropopliteal arterial lesions without severe calcification were included in this study. The study was conducted in accordance with the International Conference on Harmonization (ICH) Guidelines-Good Clinical Practices (GCP), Declaration of Helsinki, ISO 14155-1, ISO 14155-2 and Scientific Review Board requirements, and all patients gave written informed consent for participation. The patients’ mean age was 73 years and 70% were female (Table 1). In total, 23 nitinol self-expanding stents were implanted in 19 lesions in 18 extremities of 17 patients. Twelve patients received single stents for single lesions in a single extremity. One patient had tandem lesions in a single extremity and received two stents, one for each lesion. One patient had separate lesions in each extremity and received single stents for each lesion. Two patients received two overlapping stents for single lesions, and one patient received three overlapping stents for a single lesion. In summary, 19 lesions were treated in this study; 16 were treated with single stents and three were treated with overlapping stents.
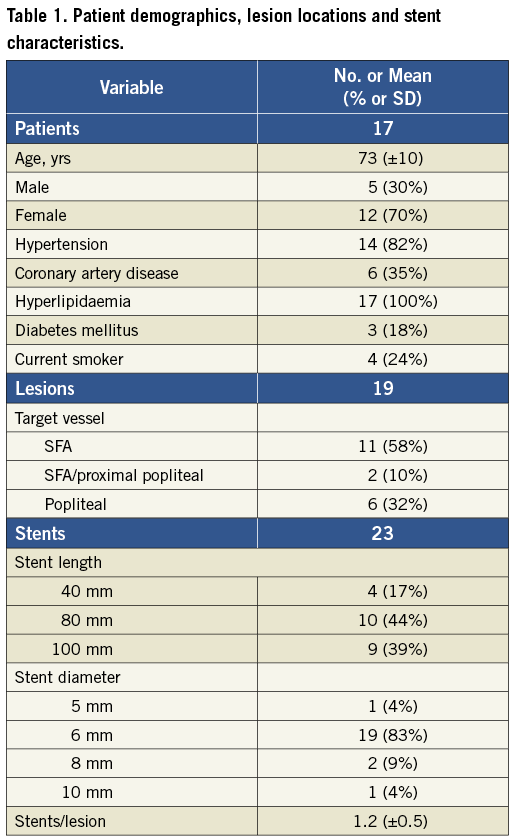
In a similar manner to a previously published study9, the femoropopliteal artery was separated into three segments for the purposes of analysis: SFA, SFA/proximal popliteal and popliteal. The boundaries of the SFA were defined (proximally) as 1 cm from the femoral bifurcation and (distally) as 3 cm from the proximal margin of the intercondylar fossa of the femur. Stents were defined as being located in the “SFA” when entirely confined to this segment, in the “SFA/prox pop” when the major portion of the stent was located in the SFA with limited extension into the popliteal artery, and in the “popliteal” when the major portion of the stent was retrogeniculate. Using this classification, stents were anatomically confined to the SFA in 11 cases, extended to the proximal popliteal artery in two cases (SFA/prox pop) and crossed the knee joint (popliteal stents) in six cases.
Two days after stent implantation, external radiopaque markers were placed on the patients’ legs and lateral view radiograms obtained with the patient standing in three different positions (Figure 1): erect (knee/hip flexion 0°/0°), in simulated ambulation (knee/hip flexion 70°/20°) and in simulated sitting or stair climbing (knee/hip flexion 90°/90°). Repeat studies were obtained after the stent had been indwelling for approximately seven months.
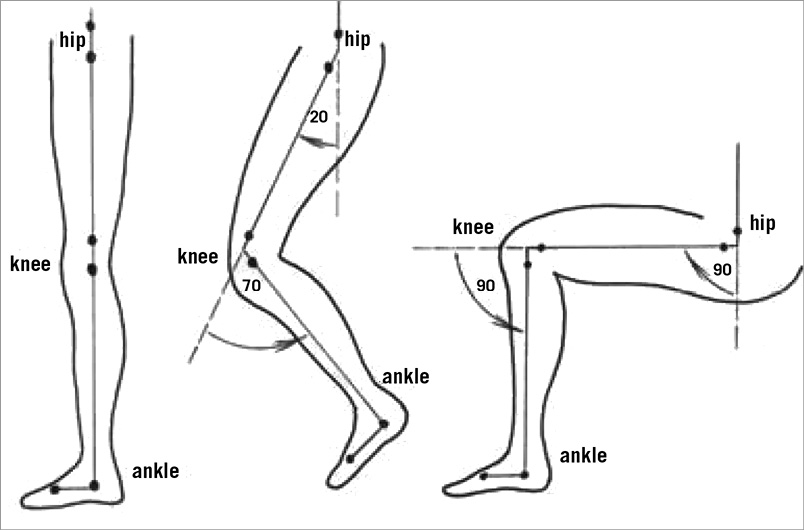
Figure 1. Leg positioning during radiographic imaging. These positions are meant to simulate standing, walking and sitting (or stair-climbing), respectively.
X-ray films were digitised and analysed with imaging analysis software (SigmaScan Pro; Systat, San Jose, CA, USA) calibrated using the radiopaque markers. The primary measurements on each film included bend angle (º) and end-to-end stent length (mm). Bend angle was measured by fitting a circle to the two tangent lines drawn from the curvature of the bent portion of the stent. The stent length was measured by drawing a centre line with multiple connecting points along the curvature of the stent, so that the total length of the line would reflect the actual stent length.
Bend angle (º) was used to describe the stent’s deviation from “straightness”. Assuming a perfectly straight stent is oriented at 180º, any stent with a bend angle <180º indicates some degree of bending. The amount of additional bending induced by flexion of the leg was defined as “deflection”. It was calculated as the difference in bend angle between the neutral and flexed positions, and expressed in degrees (º). For stents having more than one bend along their length, only the largest deflection was measured and reported. The amount of stent compression (shortening) induced by leg flexion was calculated as the difference in stent length between the neutral and flexed positions divided by the stent length in the neutral position, and expressed as percentage change in length. Examples of plain radiographs showing nitinol stents implanted in the proximal SFA and distal SFA/popliteal artery are shown in Figure 2 and Figure 3, respectively.
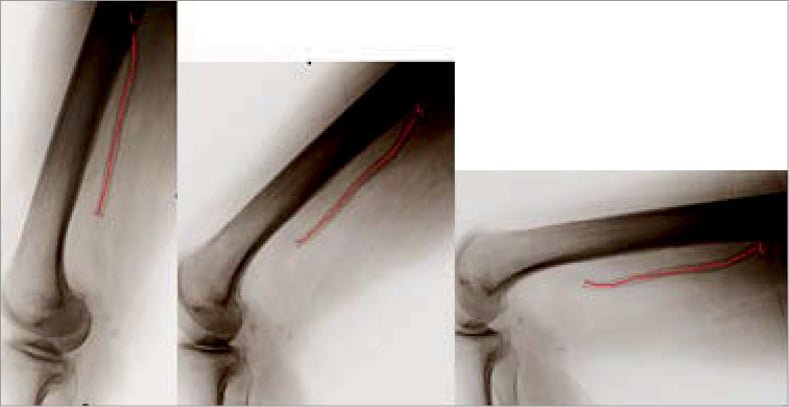
Figure 2. Self-expanding nitinol stent deformation during leg flexion. The stent has been implanted in the SFA. Three separate lateral plain radiographs show its appearance with the leg in the neutral position (left panel), 70°/20° flexion (middle panel) and 90°/90° flexion (right panel). The centreline of the stent has been marked with a red line for clarity. Note that the stent is only minimally deformed by movement of the leg. (Note also the severe calcification throughout the SFA).
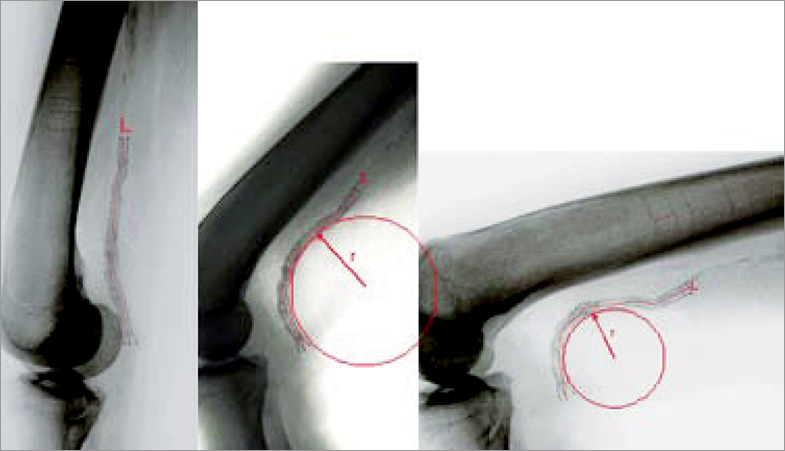
Figure 3. Self-expanding nitinol stent deformation during leg flexion. The stent has been implanted in the distal SFA and popliteal artery. Three separate lateral plain radiographs show its appearance with the leg in the neutral position (left panel), 70°/20° flexion (middle panel) and 90°/90° flexion (right panel). Note that the stent is markedly deformed by movement of the leg. The centreline (L) of the stent has been marked with a red line for clarity. The drawn circle illustrates the use of bend radius (r) to describe the degree of deformation. Stents that bend around a small circle (with a small radius) are more deformed, e.g., the more deformed stent in the right panel has a smaller bend radius than the less deformed stent in the centre panel. The nearly straight stent in the left panel has a very large bend radius that is too large to be estimated accurately.
Follow-up radiographic examinations were performed in fourteen extremities of thirteen patients after a mean follow-up of 7.1±1.3 months.
Data were entered into a standard spreadsheet for analysis (Excel 2003; Microsoft Corp., Redmond, WA, USA) and expressed as mean±SD. Student’s t-test was used for inference (GraphPad InStat; GraphPad Software, Inc., La Jolla, CA, USA) with p-values <0.05 considered statistically significant.
Results
The degree of bending sustained by nitinol stents implanted in human femoropopliteal arteries is shown in Table 2. With the leg in the neutral position, implanted stents were nearly straight, deviating from straightness by less than 30°. Flexion of the leg caused minimal additional bending of stents implanted in the SFA (5±2°), and the amount of bending was only slightly greater if the distal end of the stent terminated in the proximal popliteal artery (7±4°). As expected, leg flexion caused significant bending of stents implanted in the popliteal artery (64±16°; p<0.05 when compared to the SFA).
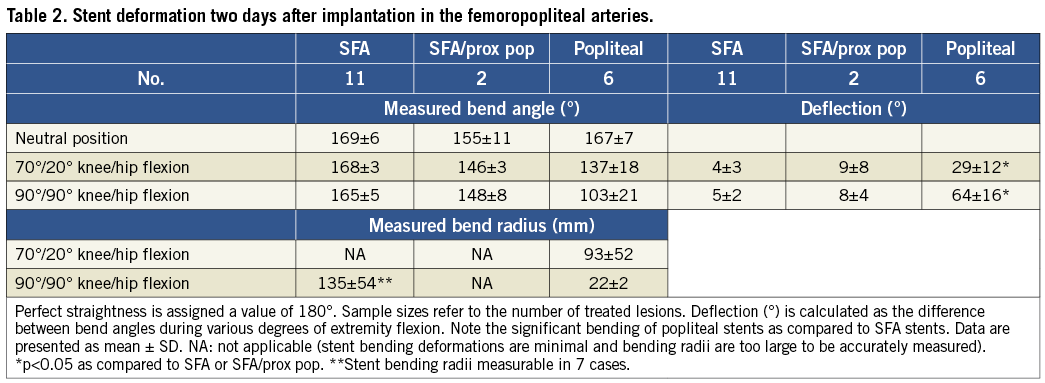
Straight leg stent length measurements correlated well with nominal stent length manufacturing specifications (Table 3). Regarding compression, nitinol stents implanted in the SFA were compressed an average of 1.7% when the leg was flexed to 70°/20°, and 3.1% when the leg was maximally flexed to 90°/90°. The maximal amount of compression observed in any case was 6.8%. When SFA stents were implanted more distally, terminating in the proximal popliteal artery, mean compression was slightly greater (5.3% vs. 3.1%) although the difference did not reach statistical significance. As expected, stent compression during leg flexion was greater for devices implanted into the popliteal artery (8.5±3.2%) as compared to the SFA (3.1±1.8%; p<0.05). There was no obvious correlation between stent length and degree of compression.
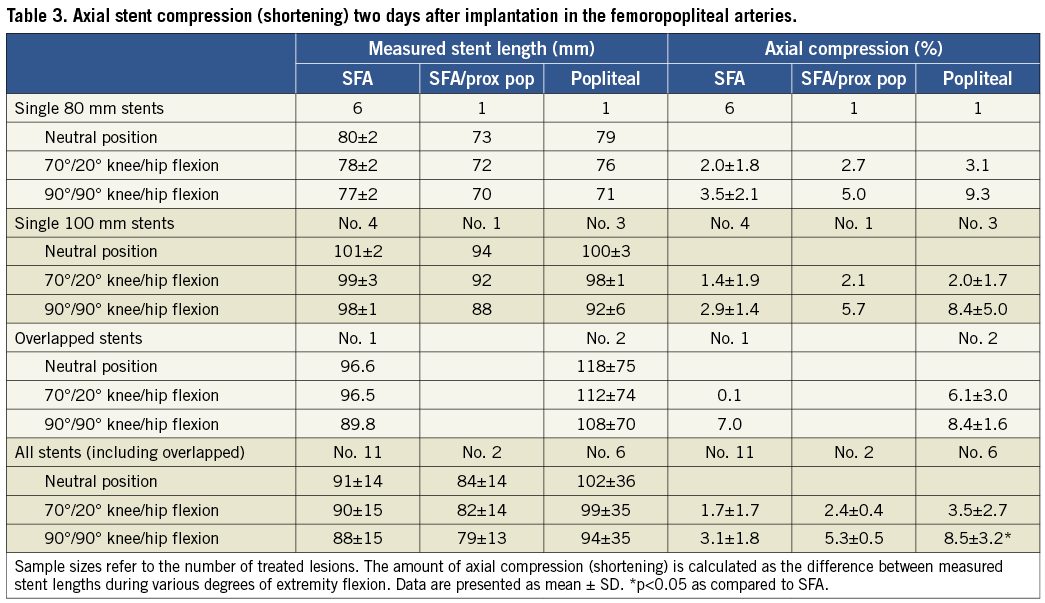
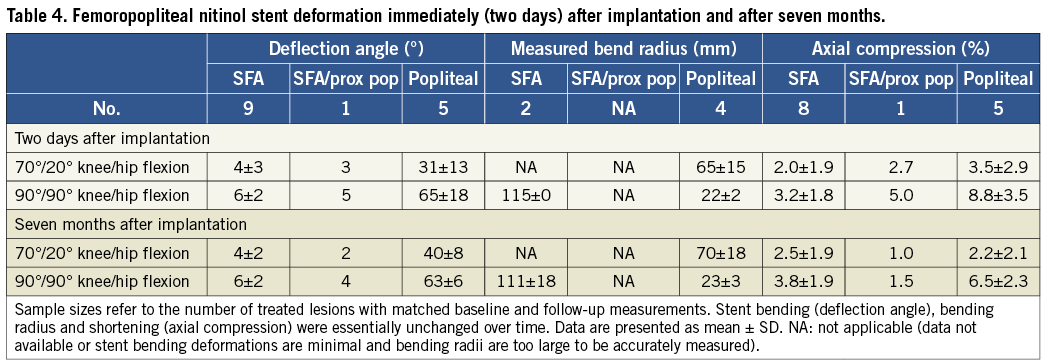
Follow-up examinations were performed in fourteen extremities of thirteen patients after a mean follow-up of 7.1±1.3 months. In these patients, the degree of stent deformation during leg flexion was essentially unchanged as compared to immediate post-procedure levels (Table 4). No obvious stent fractures were detected.
Discussion
Revascularisation of the ischaemic lower extremity, once approachable only via open surgical bypass grafting, is now increasingly amenable to minimally invasive endovascular techniques. Following its introduction in 198211, peripheral endovascular intervention has been rapidly refined over the last thirty years, including significant improvements in access, imaging, lesion traversal and, most notably, a broader application of self-expanding nitinol stent technology12-14. The recently published “Trans-Atlantic Inter-Society Consensus (TASC) Document on Management of Peripheral Arterial Disease” now recommends endovascular therapy for all ischaemic patients with segmental femoropopliteal disease <15 cm, and for selected patients with more extensive disease carrying prohibitive operative risk15. Indeed, due to the fact that a failed endovascular procedure rarely precludes subsequent attempts at salvage bypass, some centres are now employing the strategy of primary endovascular revascularisation for all symptomatic patients16-18.
Despite its impressive evolution, several significant drawbacks of femoropopliteal endovascular therapy remain. The most worrisome is the disquieting frequency of strut fracture. Many interventionists have observed this phenomenon19-26, with the incidence reaching an alarming 65% in one clinical report7. Stent fracture occurs after implantation in other anatomic areas as well, but the reported incidences are low and clinical sequelae rare1,3,21. Fracture has occasionally been observed after stent implantation in the popliteal artery, but the number of reported incidents is low as most practitioners avoid placing stents behind the knee27,28.
It has been theorised by many that the frequent fracture of stents implanted in the SFA is probably due to the unique and profound forces to which the device is exposed8,10,22,29-35. Indeed, due to the relatively fixed nature of the femoral artery in the groin and the tibial arteries in the calf, the SFA bears the brunt of absorbing most of the motion of the axial skeleton. For instance, Cheng et al quantified in vivo arterial deformation using magnetic resonance angiography and found that, during movement from the supine to the foetal position, the SFA shortened an average of 13% and twisted an average of 60°10. A subsequent study in elderly subjects found lesser degrees of shortening with flexion, but substantially more curvature and buckling31. Similarly, Klein et al studied SFA deformation in patients with PVD using an angiography-based three-dimensional model, and showed that the act of crossing the legs caused the SFA to shorten by 6.1%, bend by 0.11 cm–1 and twist by 0.006 cm–1 33,34,36. More severe deformation was observed in the popliteal artery, including curvature of 0.2 cm–1 which, when converted to mm, is strikingly similar to the level observed in the present study (50 mm vs. 55 mm).
The motion and deformation of stents implanted in actual human SFAs has also been studied, but only in cadaveric models. For instance, Nikanorov et al deployed eleven 100 mm self-expanding nitinol stents in the femoropopliteal arteries of eight cadavers and, similar to the present study, assessed length and deflection via lateral view radiographs obtained during flexion8,9. The results showed that stents implanted in the SFA, SFA/prox pop and popliteal arteries exhibited compression of 3.4%-10.7%, depending on the degree of flexion. Only minimal bending of stents implanted in the SFA and SFA/prox pop was observed (4º and 15º, respectively). However, stents implanted in the popliteal artery bent an average of 54º when the leg was fully flexed.
The present study is the first, to our knowledge, to examine the deformation of peripheral intravascular stents implanted in affected patients. Twenty-three nitinol self-expanding stents were implanted in seventeen patients with symptomatic PVD and, similar to the cadaver study cited above, stent deformation during active motion was assessed by geometric measurements made on lateral view radiographs. The results were strikingly similar to previous studies. Stents implanted in the SFA exhibited minimal bending and compression during flexion (~15° and ~3%, respectively), while stents implanted in the popliteal arteries exhibited more pronounced deformation (bending ~65° and compression ~9%). Although study populations and methodologies differ, the available data that address the phenomenon of SFA deformation are remarkably consistent.
Perhaps the most notable finding of this study is that the degree of stent deformation with ongoing bodily movement appears to change little over time. Re-examination of implanted nitinol stents after seven months showed essentially no change in deformation when compared to post-procedure levels (Table 4). Although arterial remodelling in response to self-expanding stent implantation can be profound37-40, these data suggest that the position and morphology of the stent remain relatively constant and that the degree of chronic deformation can largely be predicted by post-procedure assessment. To our knowledge, this is the first study that explores the character and degree of deformations in actual stented arteries in intact patients and how deformations might change over time.
Limitations
Several limitations to the findings presented in this study should be noted. First, the study involved a relatively small sample of highly selected patients and was observational in nature. Patients were asked to bend their legs while standing in order to simulate sitting and ambulation, but they were not actually sitting or walking. Plain radiographs are two-dimensional in nature, so they do not allow analysis of bending in three-dimensional space. This has been well documented in stents implanted in this location10. Lastly, and most importantly, only a single stent design was tested in this study (Absolute), given the specific interest and employment of several of the authors. The Absolute stent employs a 0.008” strut thickness design based upon a series of sinusoidal rings that are connected at six locations around the circumference such that the connections are aligned along the length of the stent and positioned 60º from each other. The result is a highly flexible device that maintains its expanded cross-sectional shape inside tortuous arteries, and is resistant to fatigue following repetitive deformation9. However, many other nitinol stent designs are available, each with a unique profile of mechanical properties and performance41,42. Whether other stents would exhibit the same results and constancy remains unknown.
Conclusions
In summary, this is the first study, to our knowledge, that quantifies nitinol stent deformation after implantation into actual patients with PVD. The findings demonstrated that indwelling self-expanding nitinol stents are minimally bent and compressed when implanted into the SFA (bending ~7° and compression ~5% during leg flexion). When implanted into the popliteal artery, however, the devices are deformed more severely (bending ~60° and compression ~8%). Interestingly, the degree of deformation observed immediately after implantation appears to be predictive of the chronic state, as repeat measurements obtained after a mean of seven months were essentially unchanged from baseline. It is hoped that morphometric data such as these can serve as a guide for the design and development of additional safe and effective devices for endovascular intervention.
Conflict of interest statement
A. Nikanorov, H. Zhao and L.B. Schwartz are all full-time employees of Abbott Laboratories. M. Schillinger serves on the advisory board of Abbott Vascular. E. Minar has no conflicts of interest to declare.

