Abstract
Aims: The histologic response to self-expanding stent implantation into advanced atherosclerotic lesions has not been systematically investigated. We tested the hypothesis of whether gradual expansion of advanced atherosclerotic plaques by self-expanding stents would be an appropriate method to seal atherosclerotic lesions without causing plaque disruption as is usually observed with balloon-expandable stents.
Methods and results: Twelve New Zealand white rabbits were fed an atherogenic diet (1% cholesterol) followed by arterial denudation and injection of washed autologous erythrocytes. Nitinol self-expanding stents of two different stent designs and strengths (n=12 for SX and n=12 for Micro-SX) were implanted into the previously formed lesions within the abdominal aorta six weeks following injection of erythrocytes. Four weeks following stent implantation, animals were sacrificed, specimens harvested and processed for histology. Histomorphometry was performed on stented and adjacent non-stented regions. Atherosclerotic lesions were composed of foam cells, cholesterol clefts and necrotic plaque. While SX stents showed an unfavourable outcome with respect to vessel remodelling and the percentage of uncovered stent struts, Micro-SX stents had fewer uncovered stent struts, less positive remodelling and less plaque injury.
Conclusions: Nitinol Micro-SX self-expanding stents might be a valuable approach to seal high risk atherosclerotic lesions.
Introduction
Patients with acute coronary syndromes (ACS) present with unstable angina, acute myocardial infarction (AMI), either as non-ST elevation (NSTEMI) or ST elevation myocardial infarction (STEMI), and sudden coronary death. Human autopsy studies have revealed acute plaque rupture as the most frequent underlying cause of ACS resulting in luminal thrombosis and accompanying ischaemia of the affected myocardial segments1-3. Other causes of ACS include plaque erosion and calcified nodules which are less frequent and commonly related to different risk factors from those seen in patients dying from acute plaque rupture1-3. Despite the widespread use of balloon expandable coronary stents as the preferential revascularisation technique in patients presenting with ACS, the vascular response to balloon versus self-expanding stent deployment remains largely unknown in this setting.
It is our experience that vascular healing following balloon expandable stent implantation at vulnerable plaque locations is significantly prolonged as compared to stable atherosclerotic lesions4. It may, therefore, be valuable to know if self-expanding stents at these vulnerable sites would result in disruption of the necrotic core.
Short of animal models reflecting the precise biological sequences of vulnerable plaques, it is impossible to know if such benefits are possible to achieve in man. We have previously reported on an animal model that mimics the progression of atherosclerotic plaque by inducing plaque-related haemorrhage with enlargement of necrotic core, macrophage infiltration, and iron deposition5. The purpose of the current study was to test the hypothesis if gradual expansion of advanced atherosclerotic plaques by self-expanding stents would be an appropriate way to seal these lesions without causing plaque disruption known from stenting atherosclerotic lesions with balloon-expandable stents.
Methods
This protocol was approved by the Institutional Animal Care and Use Committee of the Medstar Research Institute and conformed to the position of the American Heart Association on use of animals in research and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Rabbit model of simulated intraplaque haemorrhage
Twelve New Zealand white rabbits, 3-4 months old, were fed an atherogenic diet containing 1 percent cholesterol and 6 percent peanut oil for a total of five weeks. After one week of the high-cholesterol diet, balloon injury was carried out in the abdominal aorta as previously reported5. Subsequently, animals were switched to a low-cholesterol containing diet (0.025%) for the remainder of the study. After eight weeks of the low-cholesterol diet, the rabbits underwent injection of washed autologous erythrocytes directly into the atherosclerotic lesions within the abdominal aorta as previously reported, and maintained on low cholesterol diet for another 4 weeks5 (Figure 1).
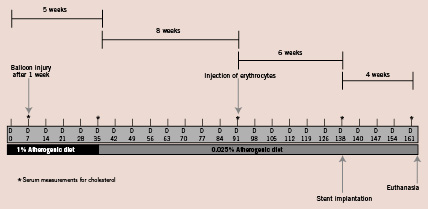
Figure 1. Study flow chart showing the temporal sequences of interventional procedures, high cholesterol diet and injection of erythrocytes into the atherosclerotic vessel wall in rabbits.
Stent design and preparation
The SX and Micro-SX stents were both designed to allow for deployment and placement without the necessity of balloon dilation, therefore, minimising acute trauma experienced at the site of balloon expandable stents. The SX and Micro-SX architectures represent different possible extremes in designing a self-expanding stent for high risk atherosclerotic coronary lesions. The SX stent was intended to provide the maximum outward strength possible for a coronary sized nitinol stent, while the Micro-SX stent was intended to provide the minimum outward strength required to assure the device would not migrate after deployment. The SX stent provided uniform scaffolding coverage with a total of thirty struts (strut thickness 99 µm) around the circumference of the design, each 1.3 mm in length, providing a vessel wall coverage of 19.2±2.9%. The Micro-SX stent offered comparatively less scaffolding coverage, with a total of fourteen struts (strut thickness 96 µm) around the circumference of the design, each 2.5 mm in length, providing a vessel wall coverage of 25.9±2.9%. Both designs were laser microfabricated from medical grade SE508 nitinol tubing, and electropolished to provide a passivated titanium oxide rich surface finish comparable to that of commercially available nitinol cardiovascular implants. The compression force of the SX stent was measured as 0.14 N/mm at a diameter of 4 mm, while that one of the Micro-SX was 0.09 N/mm. Both stents were designed to expand to a dimension of 4.8 mm. The stents were cleaned and loaded into a custom retractable sheath delivery system designed to accommodate either implant design. The loaded systems were packaged and sterilised with ethylene oxide using techniques similar to those used for commercial devices.
Stent placement, erythrocyte injection and tissue harvest
All surgery was performed using aseptic techniques. General anaesthesia was induced using ketamine (25 mg/kg), xylazine (2.5 mg/kg), and acepromazine (0.2 mg/kg). Endotracheal intubation was performed, ventilation was initiated, and anaesthesia was maintained with 3% isoflurane. Nitinol self-expanding stents were implanted six weeks following injections of autologous erythrocytes into the abdominal aorta. The stents were pre-loaded in delivery catheters with proximal and distal markers indicating each end of the stent. The delivery catheter, together with the constrained implant, was carefully advanced in small increments over the guidewire. The remainder of the delivery catheter was advanced into the atherosclerotic lesion using fluoroscopic guidance and implants deployed by retraction of the protective sheath, which allowed the superelastic shape recovery expansion of the stent for placement in the vessel.
Nitinol self-expanding stents (SX, 4.1x17 mm nitinol stent; Micro-SX, 4.1x24 mm nitinol stent of minimal strength and low profile) were consecutively deployed within the previously created lesions in the abdominal aorta, leaving approximately 2 cm between the proximal and the distal stent. Following stent deployment, angiography was performed to document stent patency. All animals received aspirin 40 mg/d orally until euthanasia. In addition, heparin (150 IU/kg) was administered intra-arterially before catheterisation procedures. Animals were randomised to two different groups: SX stents (n=12) and Micro-SX stents (n=12). Twenty-eight days post-stenting, animals were anaesthetised, and underwent angiography followed by euthanasia and perfusion-fixation. The proximal aorta and the proximal femoral arteries were dissected and submitted for histopathologic evaluation. The arteries were radiographed and the stented segments were embedded in methylmethacrylate and after polymerisation, two to three millimetre segments were sawed from the proximal, mid and distal portions of each stent and ground to a thickness of 30-40 microns using the Exakt Linear Grinding technology. Ground sections were polished and stained with toluidine blue and basic fuchsin stains. Segments from the balloon-injured vessels, proximal and distal to the stent were embedded in paraffin, sectioned at 4 microns and stained with H&E (hematoxylin and eosin) and Movat pentachrome stains.
Data analysis
All arterial segments were examined with the observer blinded to the treatment group and evaluated for thrombosis, dissection, disruption or laceration of atherosclerotic plaque, as well as the presence of necrosis. Computerised planimetry was performed (IP Lab, Scanalytics, BD Biosciences, Rockville, MD, USA) on all stented as well as proximal and distal non-stented sections as previously described6. Fibrin was identified as intense, homogeneous pink stain and semi-quantified as described previously6. The neointimal inflammation score was assessed as previously described7, and the percentage of the stent struts surrounded by giant cells was semi-quantified and compared among groups. Plaque necrosis in stented and non-stented sections was defined as heterogeneous, acellular areas, rich in cholesterol clefts and cellular debris, mostly arising form apoptotic macrophages.
To efficiently characterise the influence of the different self-expanding stents on plaque remodelling, the area of atherosclerotic plaque with presence or absence of necrosis was digitally measured within the different morphological layers of the stented and non-stented arterial sections. At 200x magnification, local clusters of foam cells, cholesterol clefts and necrotic cores were manually traced within the neointimal, intimal and medial layer (Figure 2), respectively, and expressed as mean percentage area occupied by atherosclerotic plaque and necrosis area, respectively.
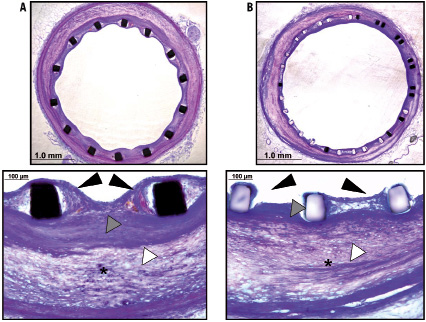
Figure 2. Histologic comparison of Micro-SX (A) and SX (B) self-expanding stents 28 days following implantation. There is good neointimal coverage and overlying endothelialisation (black arrowheads) observed in the Micro-SX stent. On the other hand, the SX group shows presence of uncovered stent struts and greater positive vessel remodelling. Atherosclerotic plaque burden was similar among groups (white arrowheads), however, there is greater plaque compression (*) in the SX group. Also, the neointima shows a smooth muscle cell-rich layer underneath the stent struts in the Micro-SX group, which likely provided a barrier against the underlying necrotic plaque (grey arrowheads) not seen in the SX self-expanding stent.
To semi-quantify the disruption of atherosclerotic plaque following stent implantation, images were magnified at 100x and screened for discrete dissection planes, laceration or moderate to severe prolapse of atherosclerotic plaque located above the stent struts towards the lumen. A plaque injury score was assigned ranging from 0 to 3, with 0 being absence of any plaque injury, 1 being plaque injury related to 0-25% of the arterial circumference, 2 being plaque injury related to 25-75% of the arterial circumference and 3 being greater than 75% plaque injury of the arterial circumference.
To semi-quantify the enlargement of the vessel following implantation of nitinol self-expanding stents, a remodelling index was defined as the ratio of the minimal area of the external elastic lamina (EEL) within the stented sections divided by the minimal area of the EEL within the proximal and distal non-stented sections (control regions) .
Statistical analysis
Numerical data are presented as mean_SD. Continuous variables were first checked for normal-distribution by Shapiro-Wilk-Goodness-of-fit test and student’s t-test or Wilcoxon rank-sum test performed where appropriate. A p-value <0.05 was considered statistically significant.
Results
Histologic characterisation of atherosclerotic plaque in stented and control lesions
Rabbit atheromas with injected erythrocytes were predominantly composed of lipid-laden macrophages, foam cells and cholesterol clefts (Figure 3). The deeper plaque regions also showed hemosiderin-laden macrophages as a result of breakdown of haemoglobin from the injected erythrocytes (Figure 3). Few sections showed evidence of necrosis, with absence of cells and a predominance of proteoglycans with cholesterol clefts (Figure 3).
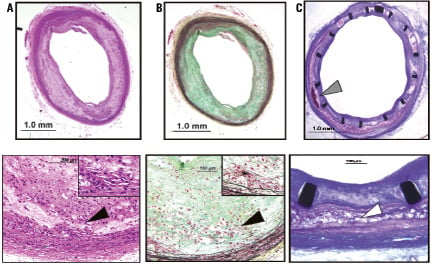
Figure 3. Photomicrographs of atherosclerotic plaques are shown in control (A and B) and stented (C) sections. Control sections are shown as H &E (A) and Movat Pentachrome (B) stained sections, while the stented lesion (C) is shown following a toluidine blue and basic fuchsin stain. Atherosclerotic plaque with presence of cholesterol clefts and areas of necrosis (*, inlet) is predominantly seen within the deeper intimal layer (black arrowheads) in control lesions following arterial denudation, while the burden of cholesterol clefts and necrosis within the intimal layer (white arrowhead) within the stented segment shows greater compression of the plaque and the medial wall; note presence of calcification within the plaque following erythrocyte injection (grey arrowhead).
Distinct dissection planes were observed within the medial layer including circumferentially infiltrating macrophages as previously described in this animal model5. The regions of erythrocyte injection showed greater plaque burden within the intima and media as compared to the control regions without erythrocyte injection (data not shown). The atherosclerotic plaque area was less in the SX stented sections compared to the Micro-SX stented sections (table 1), while there was no significant difference in plaque area among the proximal and distal non-stented sections.
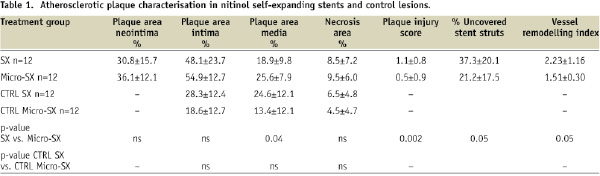
Necrotic areas were similarly distributed within the stented segments and the proximal and distal non-stented segments (Figure 3, Table 1). Atherosclerotic plaque injury and the arterial remodelling index were significantly greater in the SX treated group.
Stent deployment in advanced atherosclerotic lesions
All animals appeared healthy throughout the duration of the study. At the time point of stent implantation, serum cholesterol levels were similar among groups, with an average of 220±73 mg/dl for the SX treated group, and 235±77 mg/dl for the Micro-SX treated group (p=ns). Pre-euthanasia angiography showed widely patent stents in all treatment groups without stent migration or aneurysm formation. Stent to artery ratios were similar among groups (data not shown).
Morphometry and inflammatory response
Twelve stents from the SX treated group were compared to 12 stents of the Micro-SX treated group. The area within the external elastic lamina (EEL) and stent area were significantly greater in the SX group, while no significant difference was observed within the area of the external elastic lamina of proximal and distal non-stented sections (Table 2). Arterial injury was mild and similar among groups (mean injury scores <1) (data not shown).
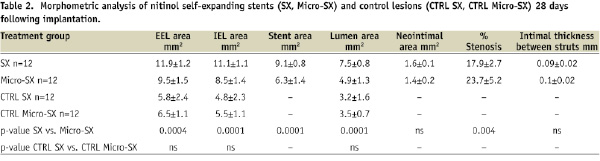
Treatment with SX stents resulted in a 24% decrease in percent stenosis compared to Micro-SX stents (Table 2), while neointimal thickness was similar among groups (0.09±0.02 for SX stents vs. 0.1±0.02 for Micro-SX stents, p=ns). On the other hand, treatment with SX stents resulted in a significant increase in the percentage of uncovered stent struts (37.3±20.1 for SX vs. 21.2±17.5 for Micro-SX stents, p=0.05) in combination with significantly enlarged vessels (remodelling index 2.2 vs. 1.5 for SX vs. Micro-SX, p=0.05). No significant differences were observed in the percentage of stent struts surrounded by fibrin, fibrin score, inflammation score, and the percentage of stent struts surrounded by giant cells (Table 3, Figure 2).
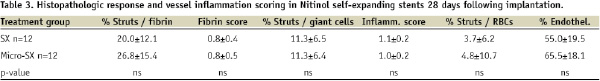
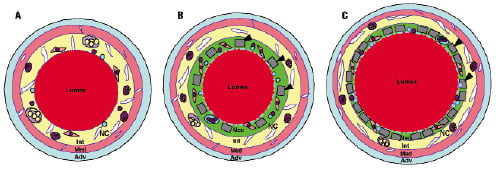
Figure 4. Schematic diagram illustrating the anatomy of a non-stented control (A), a Micro-SX stented (B) and a SX stented (C) artery. The different morphological layers are depicted as Adv (Adventitia), Med (Media), Int (Intima), and Neo (Neointima). The formation of the intimal layer is the result of arterial denudation, high cholesterol diet and erythrocyte injection into atherosclerotic lesions. The formation of the neointimal layer is a result of stent implantation. Stent struts are depicted as grey bars (black arrowheads). Monocytes are depicted as dark purple, neutrophils as blue circles, and hemosiderin- or lipid-laden macrophages as purple circles, with red or yellow dots, respectively. Smooth muscle cells are seen as pink elongated spindle-shaped cells with dark nuclei, while the proteoglycan matrix is coloured green. Foam cells are shown as enlarged oval light purple circles containing yellow globules. A giant cell surrounding stent struts is labelled with an asterisk. There is outward remodelling observed in the SX stented artery, which resulted in vessel enlargement. The necrotic core, depicted as yellow area labelled NC, is located predominantly within the intimal layer and the foam cells often extend into the media. Note: there is greater plaque and media compression in the SX vs. the Micro-SX stented arteries.
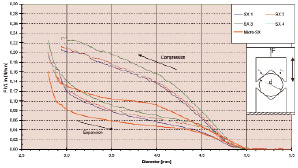
Figure 5. Diagram showing the measurements of radial strength forces. Prior to the assessment of the radial strength, both stents were measured in length utilising a calipre. The diameter of the stents were measured with the help of a 2-axis laser scanner. Three individual measurements were performed for each stent. The radial strength was measured utilising a tensile testing machine (ZWICK TM Z030/TH) at a temperature of 37º C. Prior to measurements, the stents were placed in the wide opened fixture, which allowed the testing of both expansion and compression forces by opening and closing the fixture. The absolute radial forces were normalized to the length of the stent and shown in relation to the actual diameter of the stent.
Discussion
The current study examined the potential of a previously reported animal model5 to determine the histopathologic response to stent implantation in the setting of advanced atherosclerotic lesions. Moreover, we investigated the feasibility of treating these lesions with nitinol self-expanding stents of variable design and strength. This study, for the first time, demonstrates that important histopathologic features known from human pathology following stent implantation can be methodologically recreated in an animal model of advanced atheroma formation. Moreover, it shows that low-strength self-expanding Micro-SX stents might be a valuable approach to seal advanced atherosclerotic lesions in a rabbit model of intra-plaque haemorrhage.
We have previously reported a differential response to coronary stent implantation dependent on the underlying plaque composition in a series of human autopsy cases8-13. The degree of arterial injury following stent implantation particularly depends on the composition of the underlying atherosclerotic plaque, ranging from moderate plaque stretch in fibrotic tissue to penetration of the necrotic core in lipid-rich lesions. In the current study we aimed to investigate the behaviour of nitinol self-expanding stents in atherosclerotic plaque rich in cholesterol clefts and necrotic core following injection of washed autologous erythrocytes. As previously reported, this animal model resulted in lesions rich in foamy macrophages, cholesterol clefts, and necrotic core; the three major constituents that are observed in human coronary plaques prone to rupture1-3. Implanting balloon-expandable stents into vulnerable lesions has been shown to eventually cause plaque prolapse and luminal thrombosis9,11,13. This is predominantly related to exaggerated radial forces caused by high-pressure balloons inflation and penetration of stent struts into the necrotic core.
In the current study, the low-strength nitinol Micro-SX self-expanding stent exhibited a favourable plaque remodelling profile as demonstrated by minimal vessel enlargement, low plaque injury, and less plaque compression as compared to the SX stent. Moreover, it allowed the formation of a SMC-rich tissue layer underneath the stent struts, while preserving minimal neointimal growth above.
While soft penetration of stent struts is advocated to properly secure the prosthesis to the vessel wall, excessive expansion forces can result in disruption of the underlying plaque leading to dislodgement of thrombogenic plaque components. The consequences may be often dire, with luminal thrombosis and/or distal embolisation, resulting in either acute myocardial infarction or aggravation of an already ischaemic myocardium. While soft penetration of stent struts without significant plaque shifting is observed for the Micro-SX self-expanding stent, the higher strength SX self-expanding stent showed greater plaque compression and extensive positive vessel remodelling with a higher number of stent struts not completely embedded within the surrounding tissue. It has previously been reported that stent struts lacking any neointimal coverage can be a nidus for thrombus formation, and, therefore, represent an undesirable effect14,15.
While the SX self-expanding stent showed reduced percent stenosis as compared to Micro-SX self-expanding stents, there was also extensive vessel enlargement, which is demonstrated by the larger vessel areas area in this group. The current examination demonstrates the fact that neointimal thickening can be minimised by chronic outward remodelling induced by a self-expanding stent in the presence of low vascular injury. However, too strong outward forces might also result in a delay in healing as demonstrated by the larger number of uncovered stent struts in the SX group. In contrast to other studies that utilised post-dilatation techniques to completely appose the stent16, the SX and Micro-SX self-expanding stents were deployed without the need for post-dilatation, with the reduced vascular injury that likely explains the minimal neointimal growth in both stent types.
At the same time, the Micro-SX self-expanding stent allowed a controlled neointimal growth including a balanced formation of neointima underneath the stent struts, that might represent a protective barrier against the underlying atherosclerotic plaque (Figure 2).
While proper expansion of the stent, achieved by high-pressure inflation, is encouraged for the treatment of stable atherosclerotic lesions13,17, the ideal mode of revascularisation for unstable lesions has not been fully explored. It is possible that overexpansion of balloon-expandable stents will result in deterioration of unstable plaques or even cause rupture of a thin fibrous cap when stent struts penetrate into the necrotic core. We have previously shown that penetration of stent struts into the necrotic core is the most likely cause of late stent thrombosis in bare metal as well as drug eluting stents9,13. The causal relationship between the occurrence of late thrombosis and penetration of stent struts into a necrotic core is likely to result in substantial delay in arterial healing as compared to stent struts not embedded within a necrotic core. We therefore propose that a soft gradual expansion of high-risk lesions might be more appropriate to ultimately seal these lesions without causing superficial fissure and subsequent distal embolisation of necrotic material and/or thrombus. Recently, an innovative self-expanding stent (vProtect® luminal shield; Prescient Medical, Inc., Doylestown, PA, USA) was introduced in a serious of preclinical studies evaluating the behaviour of this self-expanding stent for the purpose of treating soft atherosclerotic lesions18. Although this self-expanding stent system was not tested in atherosclerotic animals, histomorphometric evaluation showed an excellent healing profile with low inflammatory responses and complete endothelialisation by 14 days in a rabbit model. Moreover, the same stent platform was recently implanted in a human, with a very promising result at six months follow-up19.
Distal embolisation has recently been shown to be of extraordinary relevance for the periprocedural outcome in patients undergoing revascularisation in the setting of unstable angina20,21. In the study by Prati et al, reduction of plaque size measured by intravascular ultrasound (IVUS) was significantly correlated to cardiac enzyme release, and the authors hypothesised that distal embolisation of plaque material was responsible for the enzyme increase. The patients with distal embolisation are likely to have larger infarct size and declining cardiac function. In another IVUS study of patients with acute coronary syndromes, the presence of lipid-rich plaque within the stented segment was related to a transient deterioration in coronary blood flow and a higher incidence of fatal arrhythmia following PCI21. Although these studies lack a direct proof that distal embolisation of necrotic plaque material was the principal cause of the intra-myocardial coronary obstruction, these studies show remarkable parallels to the findings in recent autopsy studies that suggest luminal observation of necrotic material to be an important mechanism for the occurrence of late stent thrombosis13,22,23.
Undoubtedly, stent implantation as the preferred revascularisation procedure in patients presenting with acute coronary syndromes has shown acceptable outcomes in large randomised trials in a wide range of clinical settings24-26. However, patients presenting with ACS encompass a large variability in the underlying atheromatous lesions, with thin-cap fibroatheromas representing the majority of ACS patients. Therefore, to prevent the risk of distal embolisation in ACS patients, self-expanding stent technology may be beneficial.
In the absence of clinical trials to evaluate the behaviour of nitinol self-expanding stents in unstable lesions, preclinical studies utilising appropriate animal models remain an important means of assessing stent vessel-wall interactions in vivo.
Nitinol self-expanding stents have been reported to exert a favourable expansion profile resulting in moderate vessel remodelling and diminished neointimal growth in pig coronary arteries. Importantly, acute arterial injury was markedly reduced in nitinol self-expanding vs. balloon-expandable stents and likely contributed to the beneficial effects seen in healthy pig coronary arteries27. However, comparable experiments in atherosclerotic animal models are lacking and should provide valuable information on the behaviour of self-expanding stents in diseased lesions.
Recently, the efficacy of nitinol self-expanding stents has been established in saphenous vein graft atherosclerotic disease28. In the SCORES Saphenous Vein Graft Registry, nitinol self-expanding stents were assessed for procedural safety and device efficacy and were shown to achieve excellent intermediate outcome comparable to results known from state-of the art balloon-expandable stents in stable atherosclerotic lesions. In the current animal trial, the nitinol Micro-SX self-expanding stents were not only associated with favourable plaque remodelling, but also with minimal neointimal growth, considering that they were deployed in highly inflammatory atherosclerotic lesions.
Limitations
The current animal trial utilised an animal model that has previously been reported to mirror important vascular features of high risk plaque vulnerability. Nevertheless, it is not a model of vulnerable plaque, since human pathology of unstable lesions still differs substantially from the currently described atherosclerotic animal model. On the other hand, it is the first study to investigate the behaviour of self-expanding stents in an experimental animal model of advanced atherosclerotic disease. Therefore, the findings of the present study should impact on how we treat unstable lesions in man.
Also, no balloon-expandable control stent was used in the current study to compare and contrast the findings with the nitinol self-expanding stents. However, morphologic comparisons between balloon-expandable and self-expanding stents are difficult to make since the histopathologic response differs substantially among these stent types. Moreover, the aim of the current study was to investigate the feasibility of a novel, low-strength nitinol self-expanding Micro-SX stent, to seal an advanced atherosclerotic lesion without compromising stent efficacy in comparison to high-strength nitinol stents.
Another limitation was that no high-resolution diagnostic tools were utilised in the current study to assess correlations between histological signs of vascular healing and in vivo imaging. Nevertheless, the most relevant parameters of vascular healing were reliably evaluated with the help of dedicated histomorphometric measurements.

