
Abstract
Aims: Neoatherosclerosis is a frequent finding after implantation of permanent metallic stents. Bioresorbable scaffolds (BRS) are considered to reduce the incidence of neoatherosclerosis owing to their dissolution and consequent vascular restoration. The aim of this study was to evaluate the formation of neoatherosclerosis between magnesium-based BRS and thick-strut metallic drug-eluting stents (DES) in a rabbit model of neoatherosclerosis and in proportion to the effect of high-dose statin medication.
Methods and results: Fully bioresorbable magnesium scaffolds (BRS, n=45) and thick-strut permanent metallic DES of equivalent geometry and design (n=45) were implanted into the iliac arteries of New Zealand White rabbits (n=45) following endothelial balloon injury and exposure to a cholesterol diet. Endothelialisation was assessed in 12 animals after 35 days using scanning electron microscopy (SEM), showing significantly enhanced re-endothelialisation above struts in the BRS (n=13) compared to DES (n=10). Eleven (11) animals were terminated for baseline assessment after 91 days while the remaining 22 animals were randomised to receive high-dose statin treatment (3 mg/kg) or placebo. BRS-treated vessels showed a significant reduction in foam cell infiltration as a sign of early neoatherosclerosis by histology and OCT when compared to thick-strut DES-treated vessels. Statin treatment resulted in significant reduction of foam cell infiltration in BRS and DES by histology.
Conclusions: Our findings suggest reduced neoatherosclerosis formation in magnesium-based BRS relative to thick-strut DES. High-dose statin treatment may be a promising measure to reduce neoatherosclerosis progression, both on its own and in synergy with site-targeted device-based treatment.
Introduction
Atherothrombotic cardiovascular disease is the leading cause of morbidity and mortality worldwide1. In patients with obstructive coronary artery disease, percutaneous angioplasty and stenting is the first treatment of choice2. However, while a considerable reduction of in-stent restenosis was achieved with the introduction of drug-eluting stents (DES), the undoubted success of this technology is delivered at the collateral cost of delayed arterial healing (DAH), a pathophysiological entity which underlies a spectrum of adverse clinical events3,4. In-stent neoatherosclerosis has recently been recognised as an important disease entity in patients after stent implantation, representing an additional manifestation of atherosclerotic disease found in nascent neointimal tissue after stent implantation4.
Bioresorbable scaffolds (BRS) have recently been introduced into clinical practice. It has been proposed that their transient presence in the coronary artery may mitigate neoatherosclerosis formation. While first-generation polymer-based BRS are hampered by inherent shortcomings such as excessive strut thickness and slow degradation profile5, newer device iterations are targeted to address these limitations and provide a more rapid vascular restoration6. Furthermore, pharmacological targeting of risk factors such as hyperlipidaemia using high-dose statin treatment significantly decreased mortality in secondary prevention after manifestation of coronary artery disease7. Consequently, we aimed to investigate whether site-targeted treatment using magnesium-based BRS technology mitigates neoatherosclerosis formation in a novel hypercholesterolaemic animal model when compared to local control therapy with permanent metallic DES. It was another goal of this study to investigate the effect of BRS versus DES progression of neoatherosclerosis in proportion to systemic treatment with atorvastatin compared to placebo. The study flow is shown in Figure 1.
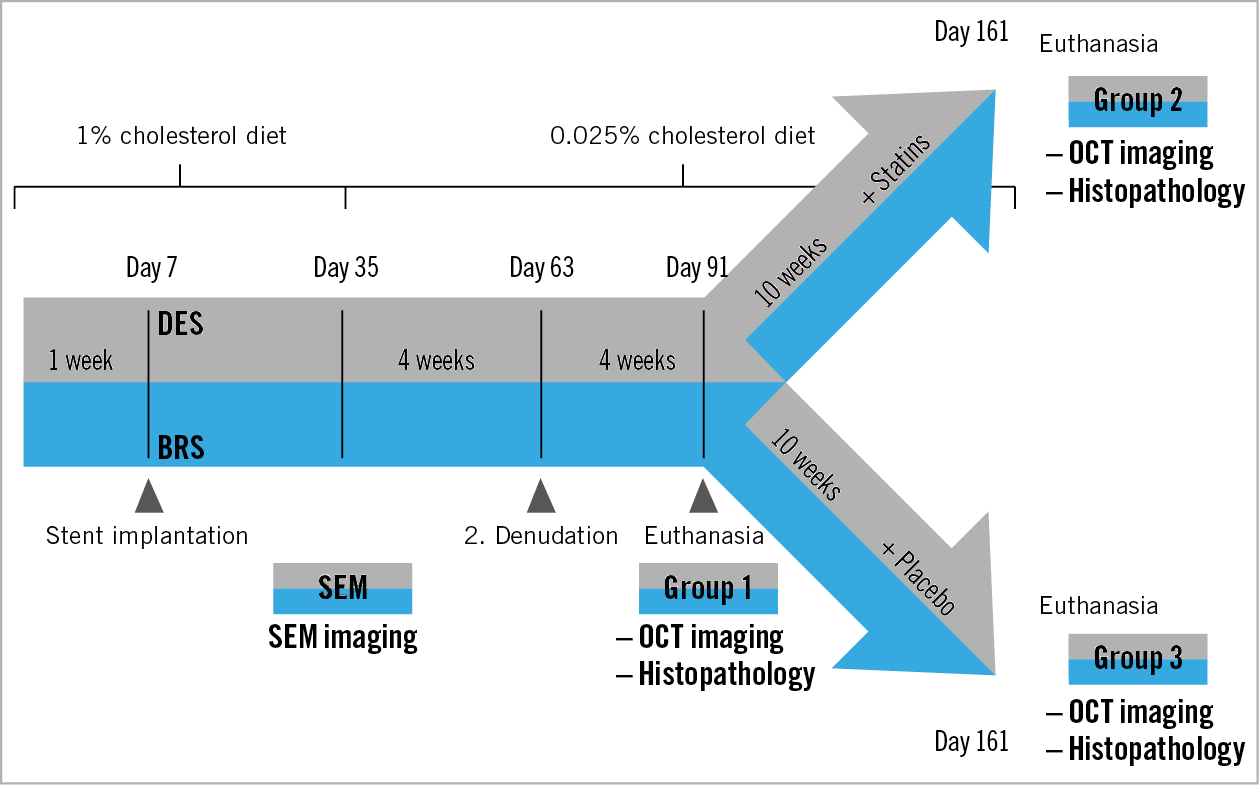
Figure 1. Study flow chart. All animals received BRS and DES in the iliac arteries seven days after the start of a 1% cholesterol diet and were switched to a low-dose cholesterol diet on day 35. Repeat balloon denudation was performed on day 63. On day 91, animals of group 1 were terminated while the remaining animals were randomised to statin treatment or placebo. The animals in groups 2 and 3 were euthanised on day 161.
Methods
Please refer to Supplementary Appendix 1 for details regarding materials and methods.
Results
Of the 33 rabbits included in the histology study, two animals died of cholesterol-induced liver failure. Two additional animals had to be excluded from analysis due to total thrombotic occlusion of a stented vessel (one rabbit with thrombosis in BRS, one with thrombosis in DES). Accordingly, a total of nine animals were available in group 1 (day 91). A total of 10 animals each remained in groups 2 and 3, respectively, until day 161.
Serum levels of cholesterol differed significantly between groups with an area under the curve (AUC) of 3608.97 in group 1 (baseline) and an AUC of 3319.28 in group 2 (statin treatment) versus 4016.92 in group 3 (placebo treatment; p=0.02) (Supplementary Figure 1).
The results of group 1 are shown in Supplementary Table 1, Supplementary Table 2, and Supplementary Appendix 2. There was no early mortality in the 12 rabbits assigned to scanning electron microscopy (SEM) assessment of endothelialisation.
NEOATHEROSCLEROSIS SCORING BY HISTOLOGY
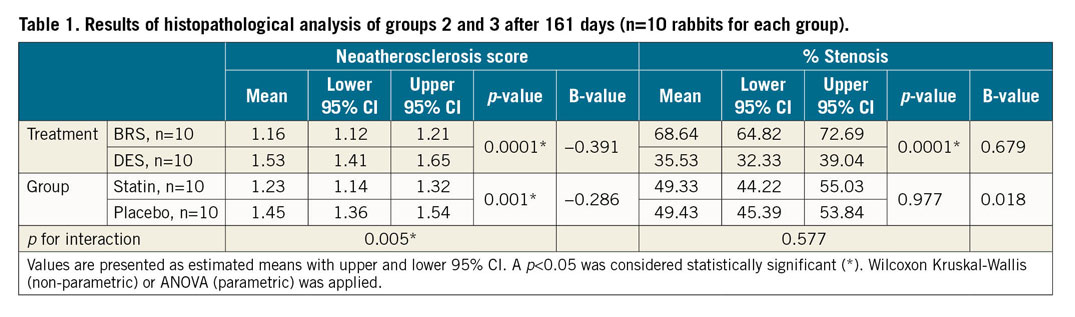
BRS showed a significantly lower estimated score of foamy macrophage infiltration compared to DES (score 1.16 [1.12, 1.21] vs 1.53 [1.41, 1.65]; mean reduction −0.361; p<0.0001) (Figure 2A). A neointimal foam cell score was additionally assigned to each quadrant of histological cross-sections, treated as a categorical variable and compared among treatment groups (BRS vs DES). Each quadrant showed significant differences based on Pearson’s chi square, with a higher percentage of foam cell score 2-4 in DES relative to BRS (p<0.05 for all quadrants).
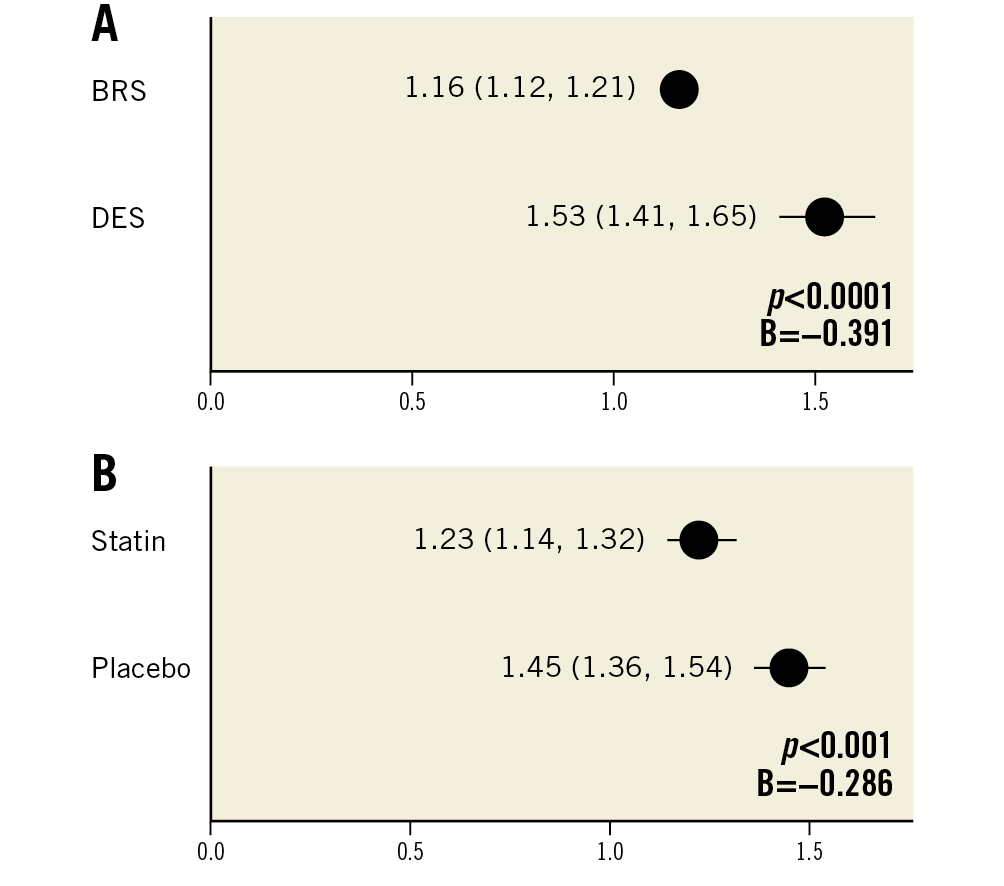
Figure 2. Neoatherosclerosis in histological sections (n=20 rabbits). Estimated mean neoatherosclerosis score derived by generalised estimating equations (lower/upper CI) shows a significant reduction of foamy macrophage infiltration in BRS as compared to DES (A) and statin-treated animals versus placebo (B).
Statin treatment resulted in a significant reduction of foamy macrophage infiltration relative to placebo (score 1.23 [1.14, 1.32] vs 1.45 [1.36, 1.54]; mean reduction −0.221; p=0.001) (Figure 2B, Table 1). Variability of scoring was assessed by calculating the intraclass and interclass correlation coefficient (0.98 and 0.90, respectively).
There was significant statistical interaction (p=0.005) among stent types and treatment allocation to statin versus placebo. Figure 3 shows representative co-registration of haematoxylin and eosin (H&E)-stained histological slides and optical coherence tomography (OCT) frames in both BRS and DES.
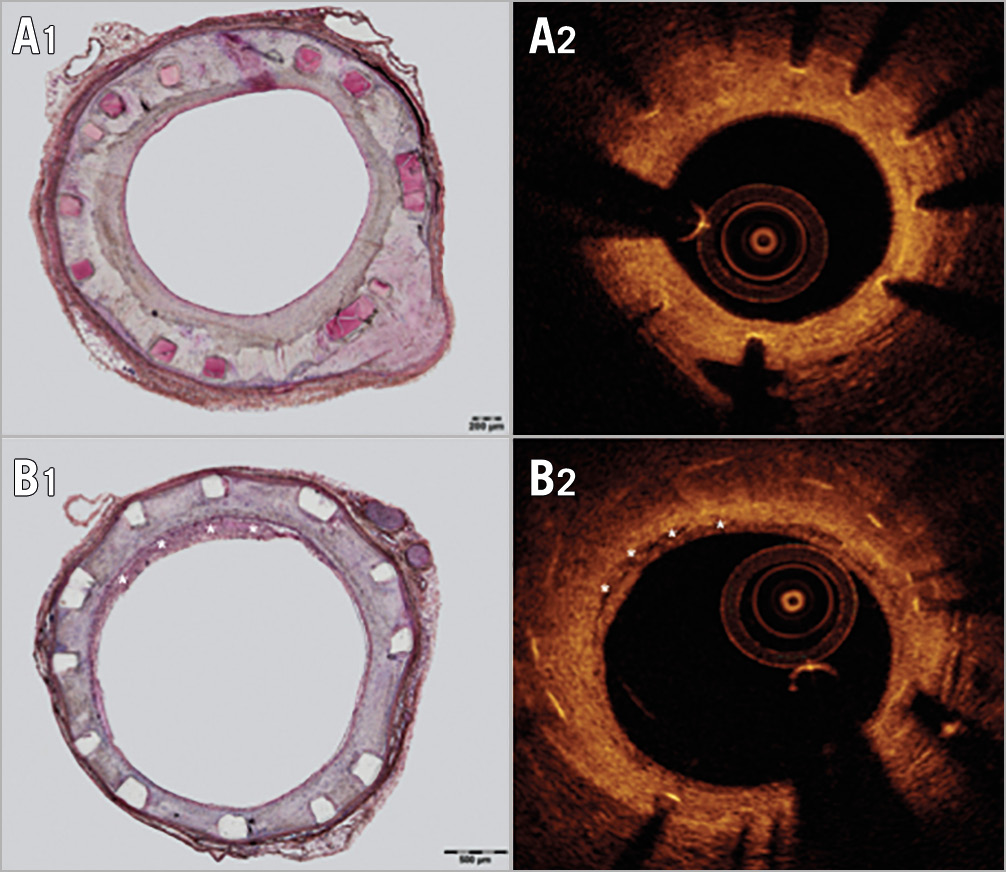
Figure 3. Comparison and co-registration of BRS and DES. A) Sections stained for H&E (A1) with corresponding OCT frame (A2) in a BRS-treated vessel. B) Sections stained for H&E (B1) with corresponding OCT frame (B2) in a DES-treated vessel. Asterisks (*) indicate foamy macrophage infiltration.
NEOATHEROSCLEROSIS SCORING BY OCT
An overview of the results is presented in Table 2. BRS showed a significantly lower estimated score of macrophage infiltration by OCT compared to DES (score 1.20 [1.08, 1.34] vs 2.09 [1.83, 2.39]; mean reduction −0.886; p<0.0001) (Figure 4A). There was no statistically significant difference in animals receiving statin treatment relative to placebo by OCT (score 1.57 [1.32, 1.87] vs 1.60 [1.44, 1.77]; mean reduction −0.26; p=0.873) (Figure 4B). Variability of scoring was assessed by calculating the intraclass and interclass correlation coefficient (0.74 and 0.69, respectively).
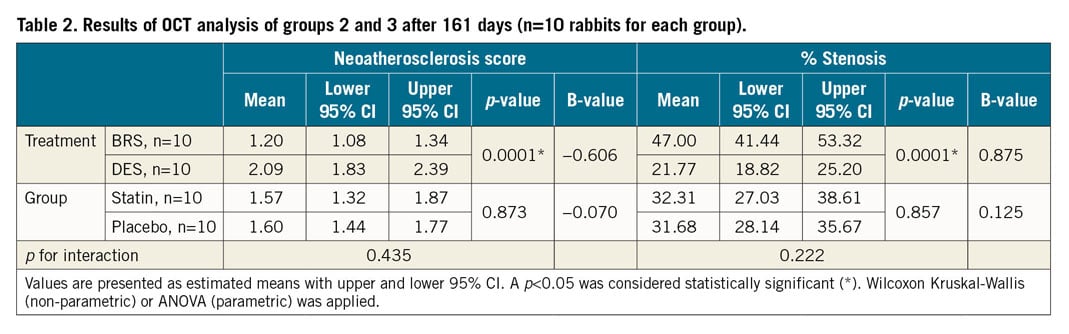
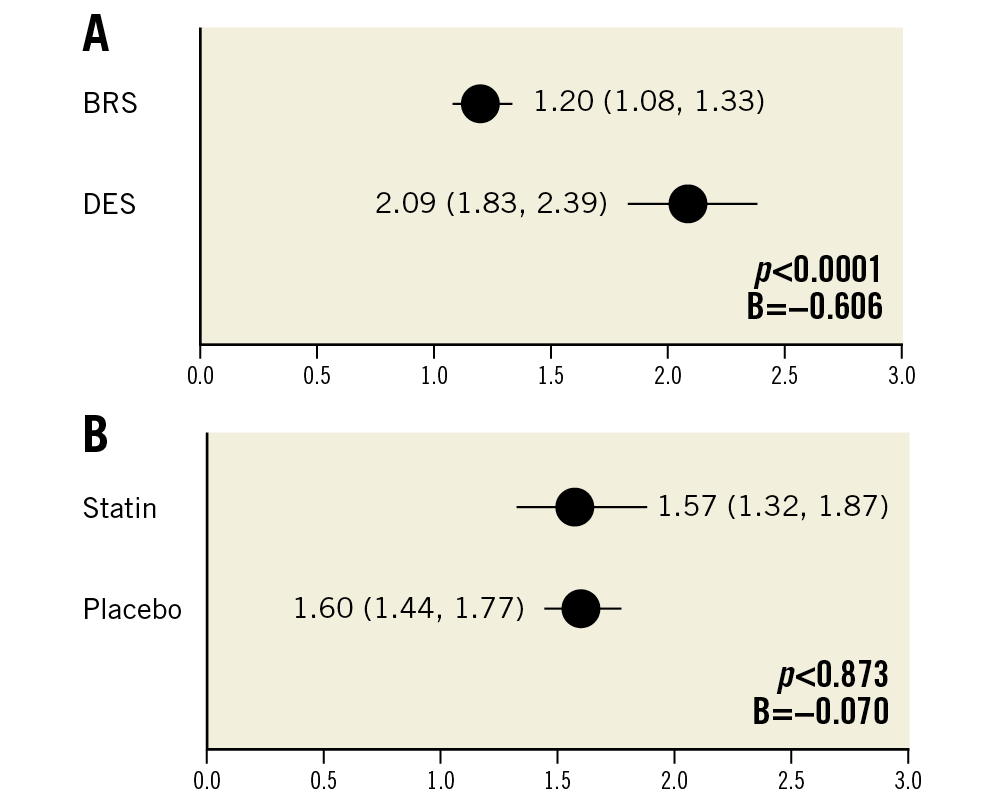
Figure 4. Neoatherosclerosis in OCT (n=20 rabbits). Estimated mean neoatherosclerosis score derived by generalised estimating equations (lower/upper CI) assessed in OCT imaging shows a significant reduction of foamy macrophage infiltration in BRS as compared to DES (A) and a non-significant reduction in the statin-treated versus the placebo group (B).
MORPHOMETRIC ANALYSIS IN OCT AND HISTOPATHOLOGY
Morphometric assessment revealed significantly greater neointimal area in BRS relative to DES by OCT (1.86 mm2 vs 1.32 mm2; mean difference +0.541 in BRS; p<0.0001) and histology (3.22 mm2 vs 2.27 mm2; mean difference +0.948 in BRS; p<0.0001). This resulted in increased percentage stenosis in BRS-treated arteries relative to DES – 47% in BRS vs 21.8% in DES (p<0.0001) in OCT analysis (Table 2) and 68.6% vs 35.5% (p<0.0001) in histological analysis (Table 1, Figure 5).
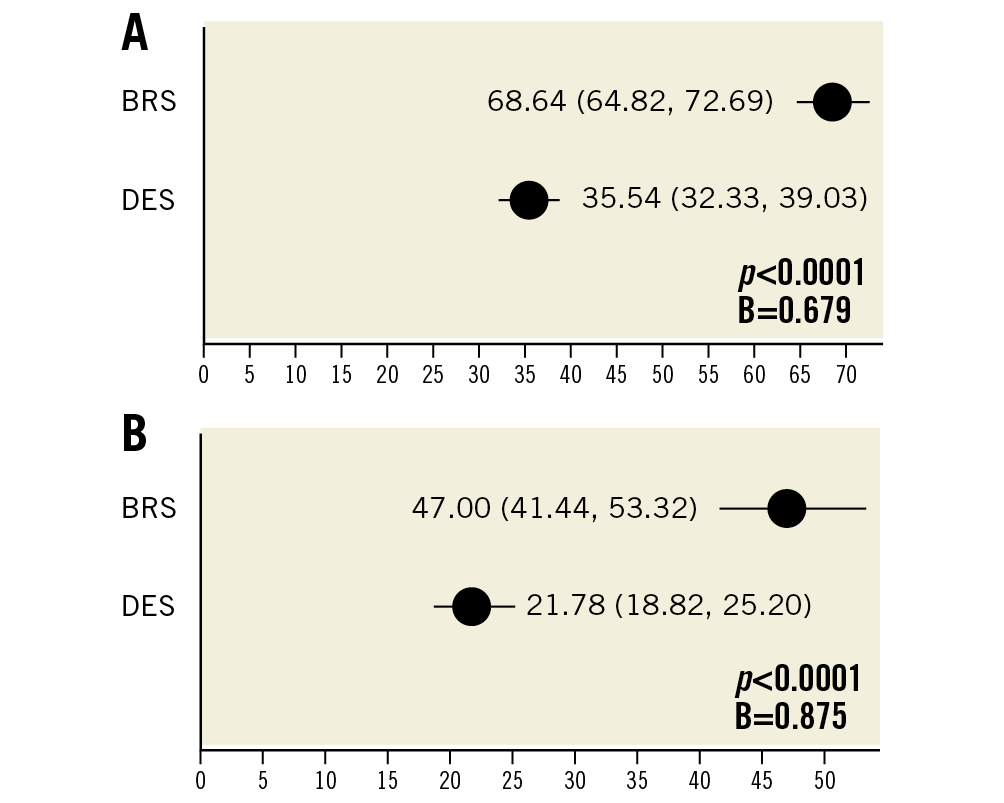
Figure 5. Percentage stenosis comparing BRS versus DES in OCT and histopathology (n=20 rabbits). A) Estimated mean percentage stenosis derived by generalised estimating equations (lower/upper CI) as assessed by morphometrical analysis of histology in both BRS and DES. B) Estimated mean percentage stenosis derived by generalised estimating equations (lower/upper CI) as assessed by OCT imaging in both BRS and DES.
HISTOLOGICAL AND IMMUNOHISTOLOGICAL STAINING
Neointimal tissue consisted of smooth muscle cells in a proteoglycan-rich matrix. Inflammation of neointimal tissue, consisting of lymphocytes and for the largest part neutrophilic granulocytes, was generally mild to moderate with a tendency towards greater inflammation in BRS relative to DES (inflammation score: 1.53 in BRS vs 1.24 in DES, p=0.106). Cholesterol crystals were frequently observed in deeper tissue layers in both BRS and DES. Giant cells were mainly observed within the peri-strut regions (% giant cells: 9.33% in BRS vs 7.70% in DES, p=0.335). In segments stained for RAM11, macrophage infiltration was frequently observed within the subintimal tissue in DES, while BRS showed scattered macrophages within the peri-strut tissue (Figure 6). Additionally, staining against smooth muscle actin (SMA) showed formation of mature smooth muscle cells in BRS (Figure 7A1, Figure 7A2), while DES did not show any positive staining for SMA (Figure 7B1, Figure 7B2). For detailed results of histopathological assessment and morphometry in histology and OCT please refer to Supplementary Table 3 and Supplementary Table 4.
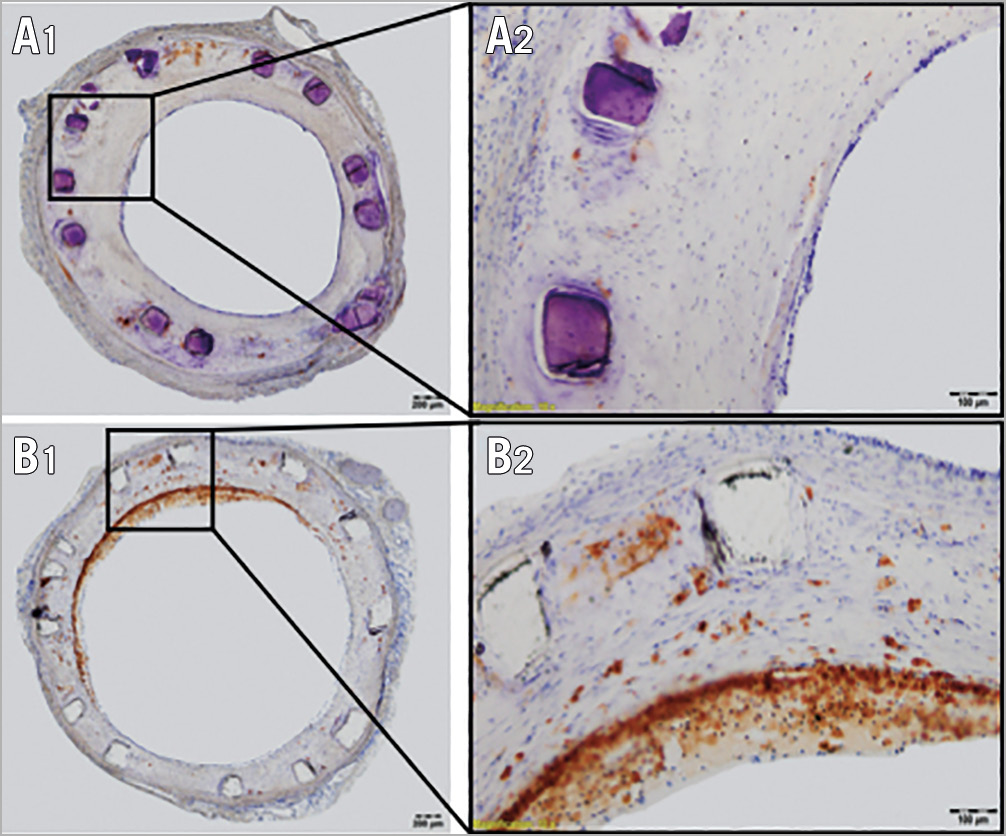
Figure 6. RAM-11 staining in BRS and DES stented vessels. A) Overview of a RAM-11 stained section from a BRS (A1) with 40x magnification (A2). Sparse macrophage infiltration is detectable in peri-strut neointimal tissue. B) Overview of a RAM-11 stained section from a DES (B1), with 40x magnification (B2). Superficial foamy macrophage infiltration is visible in about 50% of the vessel circumference.
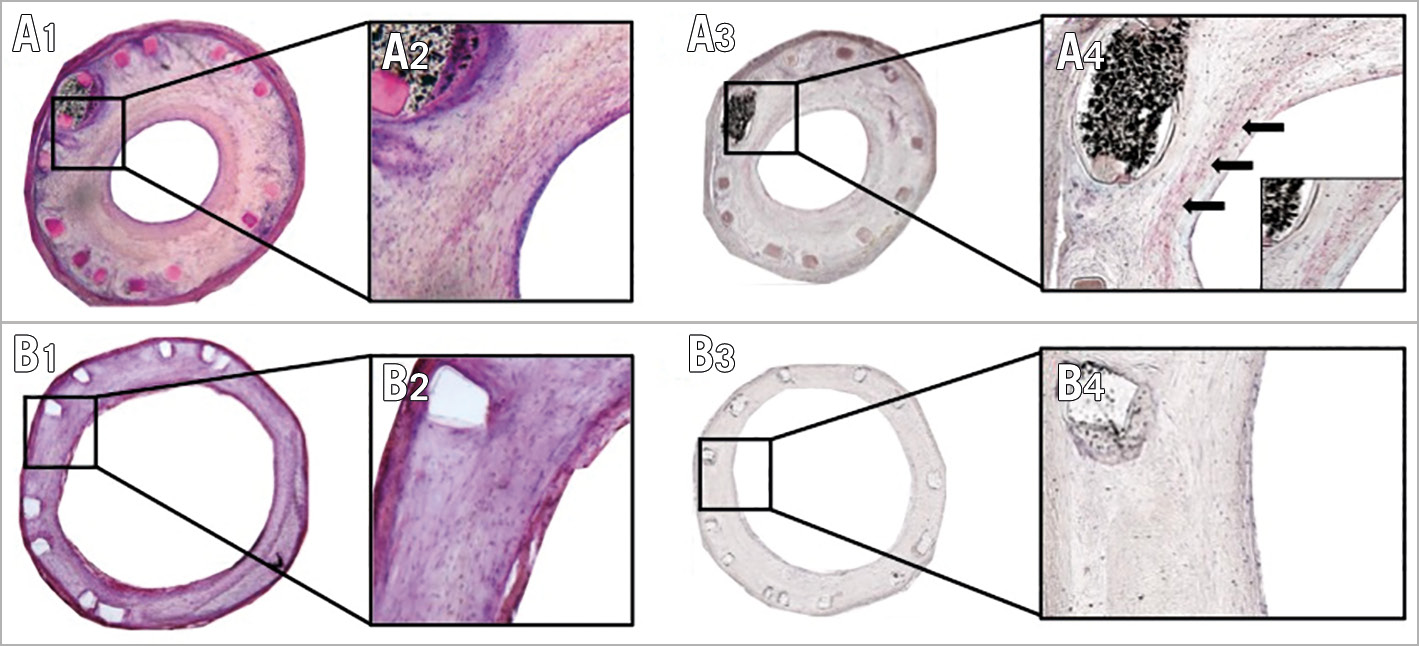
Figure 7. SMA staining in BRS and DES stented vessel. H&E stained sections in BRS (A1) and DES (B1) show the presence of spindle-shaped cells, indicating smooth muscle cells (40x magnification in A2/B2). A3) & A4) SMA stained section from a BRS with 40x magnification. Positive areas reveal these cells to be mature smooth muscle cells (arrows). B3) & B4) SMA stained section from a DES. No positive areas are visible, indicating the absence of mature smooth muscle cells.
ENDOTHELIAL CELL COVERAGE
Re-endothelialisation following catheter denudation and stenting was evaluated in 12 animals (n=13 BRS, n=10 DES) using SEM. Twenty-eight days following stent implantation (day 35), magnesium-based BRS showed significantly improved endothelial cell coverage above struts as compared to the 316L DES of equivalent design (91.7±20.2% vs 42.1±57.16%; p<0.001) (Figure 8).
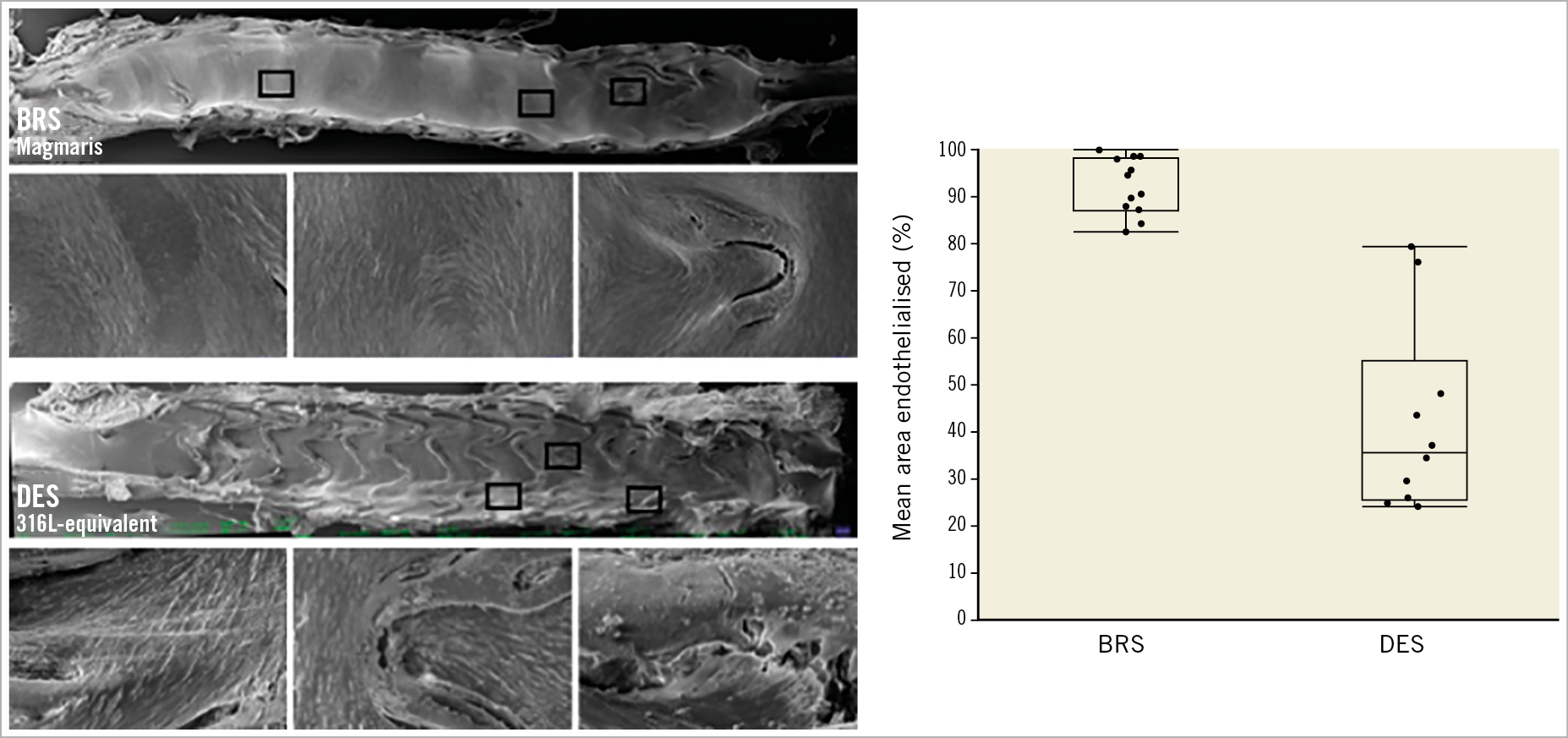
Figure 8. Endothelial cell coverage in BRS and DES. Left: scanning electron microscopy of BRS (upper panel) and DES (lower panel) with higher magnification of boxed areas. Right: quantification of endothelial cells above stent struts.
Discussion
The current study investigated the differential magnitude of neoatherosclerosis formation after implantation of fully bioresorbable magnesium scaffolds and permanent metallic thick-strut DES of equivalent design and geometry in a novel animal model of in-stent neoatherosclerosis. Furthermore, it examined the impediment of neoatherosclerosis progression as a response to statin treatment relative to placebo in both stent types up to 161 days following study initiation. The most salient findings can be described as follows:
(i) Fully bioresorbable magnesium scaffolds showed enhanced endothelial cell coverage 28 days following stent implantation and decreased neointimal macrophage infiltration after 161 days when compared to permanent stainless steel thick-strut DES in a novel animal model of neoatherosclerosis.
(ii) There was significant statistical interaction between stent type and treatment allocation to atorvastatin versus placebo with regard to reduction of neointimal macrophage infiltration. Therefore, synergistic effects between local and systemic therapy are very likely.
(iii) Atorvastatin treatment as applied in the current study significantly reduced progression of neointimal macrophage infiltration in BRS and DES, which was confirmed by histopathological assessment.
(iv) Fully bioresorbable magnesium scaffolds showed significantly greater neointima burden as compared to thick-strut DES.
Development of neoatherosclerosis after stent implantation has been recognised as an important contributor to late stent failure. The implantation of DES was recently reported to result in earlier onset of neoatherosclerosis formation in post mortem autopsy and clinical imaging studies when compared to BMS8,9. Previous work has shown that DES exhibit markedly different neointimal composition with a higher amount of proteoglycan and fewer smooth muscle cells compared with BMS10,11. Notably, long-term follow-up of clinical trials investigating the comparative performance of early- and newer-generation DES reported a sustained increase in clinical events irrespective of the implanted stent type over time12. Short of a mechanistic explanation for this late increment in adverse events, preclinical and post-mortem autopsy studies have suggested delayed arterial healing with sustained inflammatory reaction, delayed endothelialisation and formation of in-stent neoatherosclerosis to be causative in the majority of cases. Consequently, bioresorbable scaffolds were introduced to overcome these inherent limitations associated with permanent metallic DES and proposed to reduce the burden of neoatherosclerosis owing to their temporary presence, where recovery of physiological vasomotion and normalisation of flow dynamics were suggested to impact favourably on plaque progression over time. However, those anticipated effects of BRS technology have not been proven in clinical practice.
Since reliable assessment of neoatherosclerosis by OCT alone remains controversial and prospective invasive imaging studies are challenging to perform, preclinical studies remain an important means to investigate the comparative effects of medical devices and pharmacological approaches in a timely manner. We have previously validated the currently applied animal model and found excellent agreement with regard to depiction of early signs of neoatherosclerosis such as foam cell infiltration within the nascent neointima following stent implantation13. Since early stages of neoatherosclerosis can be detected reliably by OCT (i.e., macrophage infiltration), the currently applied animal model holds great potential to gain a mechanistic understanding into the pathophysiology and adoption of therapeutic strategies for the treatment of neoatherosclerosis. The application of two distinct scoring systems for detecting neoatherosclerosis could have biased our results, especially when detecting neoatherosclerosis by OCT. However, reproducibility was satisfactory and variability rather low in both scores.
Owing to the inherent design constraints of the current animal study, absolute effect size in reducing neoatherosclerosis among BRS versus DES and atorvastatin versus placebo-treated animals is difficult to ascertain. This is also reflected by significant statistical interaction among these treatment approaches with regard to reduction of neointimal macrophages. It can therefore be assumed that BRS and atorvastatin therapy resulted in synergistic effects with regard to neoatherosclerosis progression over time, resulting in reduced neointimal macrophage infiltration.
Bioresorbable scaffold technology has recently suffered a major setback after the release of randomised controlled clinical trial data, which showed significantly increased revascularisation and device thrombosis rates at longer-term follow-up in broad patient subsets14,15. Potential mechanisms of late scaffold failure have been investigated in dedicated registries using OCT evaluating the long-term outcomes of polymeric BRS to delineate vascular morphology. These suggested late strut discontinuities, malapposition, neoatherosclerosis and underexpansion of the scaffold segment to be causally associated16. While most of these findings can be attributed to scaffold dismantling over time, neoatherosclerosis is not specifically linked to bioresorbable scaffold technology, and was indeed forecast to be erased with the use of BRS.
In our study, we observed decreased foam cell infiltration after implantation of magnesium-based BRS relative to a thick-strut permanent metallic DES, which may be secondary to accelerated vascular restoration (within six months) after magnesium-based BRS implantation relative to the three-year time frame observed with first-generation polymeric BRS, return to physiologic flow conditions and accelerated recovery of functional endothelial barrier function. Drug-induced impaired re-endothelialisation is likely to play a key role in the development of neoatherosclerosis as it allows the passage of inflammatory cells and lipoproteins into the developing neointima17. Quantitative evaluation of re-endothelialisation after 28 days showed significantly improved endothelial cell coverage in BRS compared with thick-strut DES. However, the lack of sufficient evaluation of endothelial leakiness in our study is a limitation. Our data are also in contrast to previous work by Waksman showing a lower degree of endothelialisation 28 days following stent implantation18: the main difference to our study can be seen in the procedural methodology of balloon denudation and the usage of healthy versus atherosclerotic animals. Most importantly, re-endothelialisation following arterial denudation is variable and depends largely on animal species, diseased versus healthy vascular tissue conditions, and the degree of vascular injury performed prior to stent implantation. In the study by Waksman et al, standard semi-compliant balloon catheters were inflated at the target site of stent implantation in healthy rabbits to achieve endothelial denudation. In the current study, we used 3 Fr Fogarty catheters, which were inflated and repeatedly pulled back (arterial embolectomy catheters) to cause endothelial denudation in atherosclerotic rabbits. In our experience, the latter methodology causes a greater degree of endothelial denudation and vascular injury. As a consequence of greater endothelial injury in our study, proliferation and migration of endothelial cells is also markedly increased, which may explain the enhanced rate of re-endothelialisation 28 days after stent implantation. However, it is important to mention that re-endothelialisation was assessed at day 35 in this animal model, a time point at which lacking endothelial integrity is likely to impact substantially on cholesterol uptake into neointimal tissue and, consequently, formation of foamy macrophages. The fact that we observed increased foamy macrophage infiltration in thick-strut DES relative to BRS further strengthens this hypothesis. Atorvastatin has previously been proven to result in decreased plaque formation in hypercholesterolaemic rabbits19,20. There is abundant evidence of its clinical efficacy from large randomised controlled trials7. Atorvastatin was found to result in a highly significant 72% decrease of iliac-femoral lesion size following balloon injury in hypercholesterolaemic rabbits, which was attributed mainly to a significant reduction of intimal macrophages20. In a similar vein, Herdeg et al found a significant reduction in intimal and medial proliferation as well as peri-strut inflammation after atorvastatin therapy and bare metal stent implantation19, which is in line with the findings of our study, where atorvastatin therapy resulted in significantly decreased neointimal macrophage infiltration irrespective of stent type.
In our study, atorvastatin relative to placebo therapy resulted in significantly diminished progression of neointimal foam cell infiltration, which was independent from the underlying stent type.
Limitations
Firstly, animal models are never able to replicate fully all the features of certain pathologies, e.g., we only observed the early stages of neoatherosclerosis and no advanced stages such as necrotic core formation. Also, the formation of foam cells is artificially accelerated (e.g., repeat endothelial denudation) compared to human coronary atherosclerosis, which usually takes decades to develop and depends on additional important co-factors that cannot be reproduced in current animal models.
Secondly, OCT has limitations in the detection of neoatherosclerosis, as mentioned above. This can be challenging when using degrading stent struts as a hallmark to differentiate neointima from native intima in OCT and could have biased the results of neoatherosclerosis scoring. However, even after degradation, BRS struts could be reliably recognised in the majority of cases and helped to differentiate progression of atherosclerosis versus neoatherosclerosis. Additionally, the use of co-registered histology allowed reliable diagnosis of neoatherosclerosis. Thirdly, transferability of preclinical findings to a human disease state is difficult to ascertain even after applying diseased animal models.
Finally, the use of a thick-strut DES is a limitation since modern DES have much thinner struts; thus, comparison to contemporary DES used in clinical practice with respect to neoatherosclerosis remains unknown.
Consequently, the current study should be viewed as hypothesis-generating. Clinical trials designed specifically to address neoatherosclerosis formation over time are needed to confirm these findings.
Conclusion
This study is the first to suggest reduction of in-stent neoatherosclerosis in bioresorbable magnesium scaffolds compared to thick-strut DES. High-dose statin therapy may be an important secondary prevention measure in patients with confirmed neoatherosclerosis. Prospective randomised trials are needed to evaluate these preclinical findings.
|
Impact on daily practice Neoatherosclerosis was reported to contribute substantially to late stent failure, mainly caused by in-stent restenosis and stent thrombosis beyond the first year after stent implantation. In the current study, we investigated a second-generation magnesium-based BRS with regard to formation of neoatherosclerosis in hypercholesterolaemic rabbits relative to its stainless steel equivalent DES and found significantly reduced foam cell formation as an early sign of neoatherosclerosis in magnesium-based BRS. Clinical studies with appropriately designed endpoints are required to confirm these promising results in man. |
Funding
This work was supported by an European Society of Cardiology (ESC) Grant for Medical Research Innovation. Dr Katja Steiger received funding from the DFG (SFB824, Z2).
Conflict of interest statement
M. Haude reports personal fees for consulting from Biotronik, outside the submitted work. M. Joner reports grants from the ESC’s “Grants for Medical Research Innovation”, during the conduct of the study, personal fees for consulting from Biotronik and OrbusNeich, and personal fees from speaker fees from Biotronik, OrbusNeich, Boston Scientific, Medtronic, and AstraZeneca, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

