
Abstract
Aims: The MAGSTEMI trial showed larger endothelium-independent vasodilatation with magnesium-based bioresorbable scaffolds (MgBRS) than with sirolimus-eluting stents (SES). However, restenosis was more frequent with MgBRS. The aims of this study were to compare the healing pattern between MgBRS and SES and to describe the main causes of restenosis, as assessed by optical coherence tomography (OCT).
Methods and results: Ninety-five consecutive patients from the randomised MAGSTEMI trial (MgBRS=48, SES=47) underwent OCT imaging at one year. Healing and bioresorption pattern were categorised into four groups: 1) indiscernible struts were observed in 33.3% versus 0% of patients (p<0.001); 2) struts integrated into the vessel wall in 22.9% versus 63.8% (p<0.001); 3) protruding struts in 37.5% versus 31.9% (p=0.568); and 4) protruding and malapposed struts in 6.3% versus 4.3% (p=0.663), respectively. MgBRS were not suitable for strut coverage analysis; SES presented with 5.6% uncovered struts. Scaffold discontinuities were observed in 10.4% and 0%, respectively (p=0.023). MgBRS presented smaller minimal lumen area (3.92±2.02 vs 6.31±1.71 mm²; p<0.001) and larger area stenosis (52.84±18.05 vs 25.02±14.58%; p<0.001). Scaffold measurements were only feasible in 50% of MgBRS, with the expansion index being smaller than in SES (0.58±0.16 vs 0.86±0.19; p<0.001). Scaffold collapse was observed in at least 50% of cases with MgBRS restenosis.
Conclusions: Both MgBRS and SES exhibited a low degree of neointima healing, but lumen dimensions were smaller with MgBRS at one year. Although the advanced bioresorption state of MgBRS hampers the assessment of scaffold collapse, this seems to be the main mechanism of restenosis. Future generations of MgBRS should increase and prolong the radial force. Clinical trial registration: NCT03234348
Visual summary. Bioresorption and neointima patterns of Mg-based bioresorbabable scaffolds versus permanent sirolimus-eluting stents in STEMI patients at one year.
Introduction
Primary percutaneous coronary intervention (PPCI) with stent implantation is the preferred reperfusion strategy in patients with ST-segment elevation myocardial infarction (STEMI). Stents implanted in the context of PPCI have been shown to exhibit greater malapposition and higher risk of stent thrombosis than stents implanted in other clinical scenarios1,2. Moreover, STEMI patients are often younger and present with softer plaque types as compared to other clinical settings. For these reasons, STEMI is theoretically one of the best targets for bioresorbable technology. Previous experience with polymeric poly-lactic acid-based bioresorbable scaffolds has shown that they are effective in STEMI patients3,4,5.
The MAGSTEMI study (MAGnesium-based bioresorbable scaffold and vasomotor function in patients with acute ST-segment elevation myocardial infarction) is the first randomised study using the magnesium-based bioresorbable scaffold (MgBRS) (Magmaris®; Biotronik AG, Bülach, Switzerland). In this study, MgBRS showed greater endothelium-independent vasodilatation than a permanent metallic sirolimus-eluting stent (SES) (Orsiro; Biotronik AG) in STEMI patients at one-year follow-up6. However, MgBRS was associated with greater angiographic late lumen loss (0.61±0.55 mm vs 0.06±0.21 mm; p<0.001) and in-segment restenosis (20.0% vs 0%; p=0.001) than SES. The pathophysiologic mechanisms responsible for the greater lumen loss and restenosis rates in patients treated with MgBRS are still unknown.
The objectives of the present study are to describe and compare the main optical coherence tomography (OCT) parameters of stent healing between MgBRS and SES in STEMI patients at one year. This study also sought to investigate the mechanisms of angiographic in-segment MgBRS restenosis, as assessed by OCT.
Methods
POPULATION
The MAGSTEMI study (NCT03234348) is an investigator-initiated, multicentre, prospective and randomised clinical trial. This work was supported by the Spanish Heart Foundation. The design of the trial has been reported previously7. In summary, a total of 150 STEMI patients were randomised 1:1 to MgBRS or SES in 11 academic institutions. All patients were requested to undergo angiographic and vasomotor examination with nitroglycerine at one-year follow-up. The primary endpoint of the study was the observation of >3% vasodilatation within the scaffold segment7. The study was performed according to the provisions of the Declaration of Helsinki. The ethics committee of each participating centre approved the study protocol. Written informed consent was obtained from all patients.
The present investigation is a pre-specified substudy of the MAGSTEMI trial. As per protocol, three institutions (out of 11) of the MAGSTEMI trial were requested to perform OCT imaging during the one-year coronary angiography. This group of consecutive patients was classified as the “OCT per protocol group”. Moreover, all study investigators aimed to perform OCT imaging in case of angiographic in-segment restenosis (diameter stenosis ≥50%) irrespective of the study institution. This group was classified as the “OCT per restenosis group”.
OPTICAL COHERENCE TOMOGRAPHY
OCT imaging was performed after vasomotor examination with the Dragonfly™ OPTIS™ catheter (Abbott Vascular, Santa Clara, CA, USA) according to standard procedures. OCT analysis was performed by a dedicated core laboratory (BARCICORE-lab, Barcelona, Spain) using specific software for analysis (LightLab Imaging/Abbott Vascular). The analysed segment included the stent region and the stent margins defined as the vessel segment 5 mm proximal and distal to the stent.
Quantitative OCT analysis is described in Supplementary Appendix 1. Due to the advanced bioresorption state of the MgBRS, the scaffold area was clearly visible in only 40% of OCT cross-sections at one year. For this reason, all OCT variables involving scaffold measurements have been reported in patients with ≥40% of cross-sections suitable for scaffold area assessment. Stent expansion was calculated as minimal stent area/reference lumen area. Neointima area stenosis was calculated in all suitable cross-sections as neointima area/stent area *100.
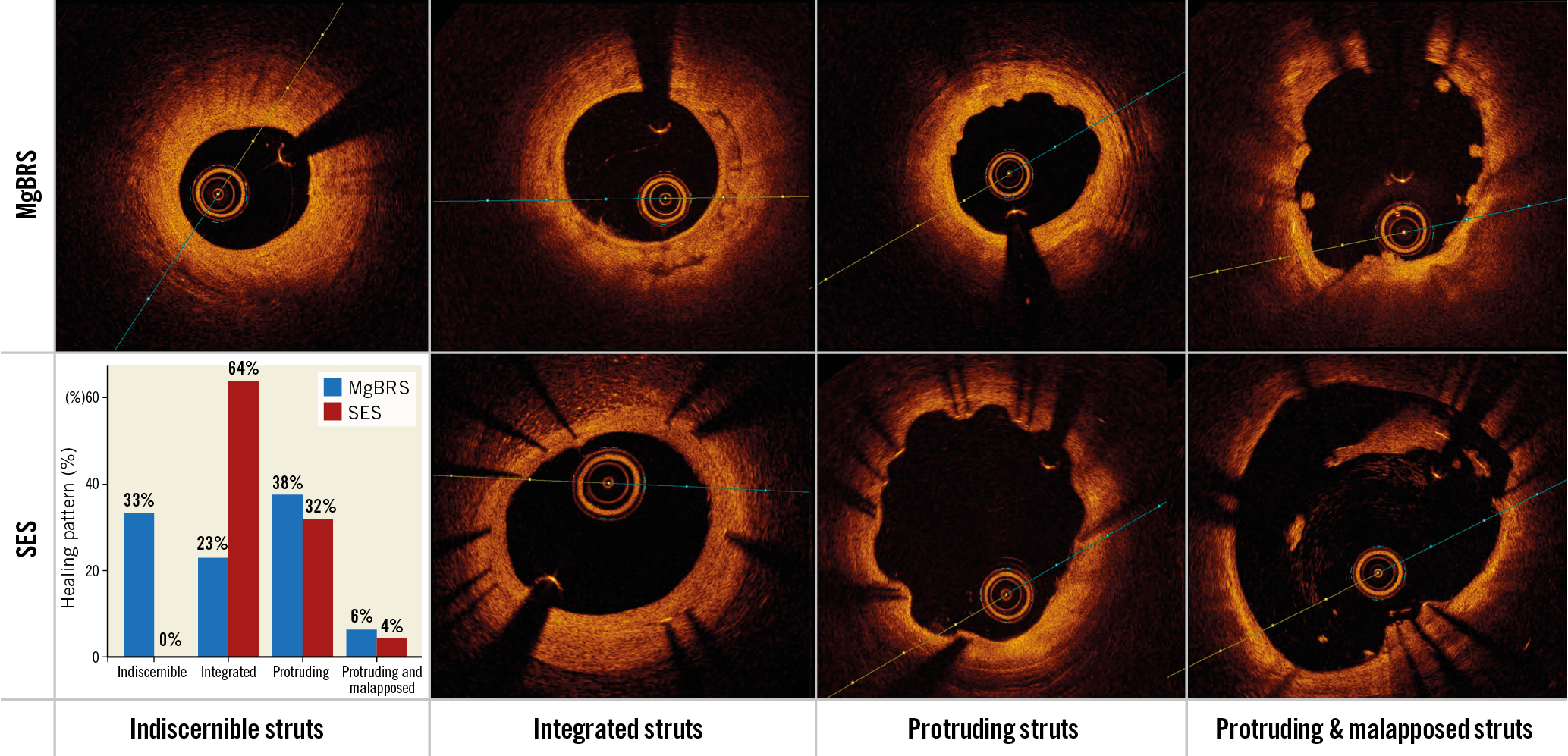
Qualitative OCT analysis was performed by agreement of two analysts (J. Gomez-Lara and L. Ortega-Paz). According to the predominant number of cross-sections with one of the four different types of bioresorption and healing states, both study devices were classified into the following groups: 1) indiscernible struts, 2) visible struts completely integrated into the vessel wall; 3) visible struts protruding into the lumen causing a characteristic bumpy contour8; and 4) visible struts protruding and malapposed to the vessel wall. The core laboratory kappa value for this qualitative finding was 0.81. Other qualitative OCT findings are described in Supplementary Appendix 2. Figure 1 and Figure 2 show examples of different qualitative OCT findings. Scaffold restenosis types are described in Supplementary Appendix 3.
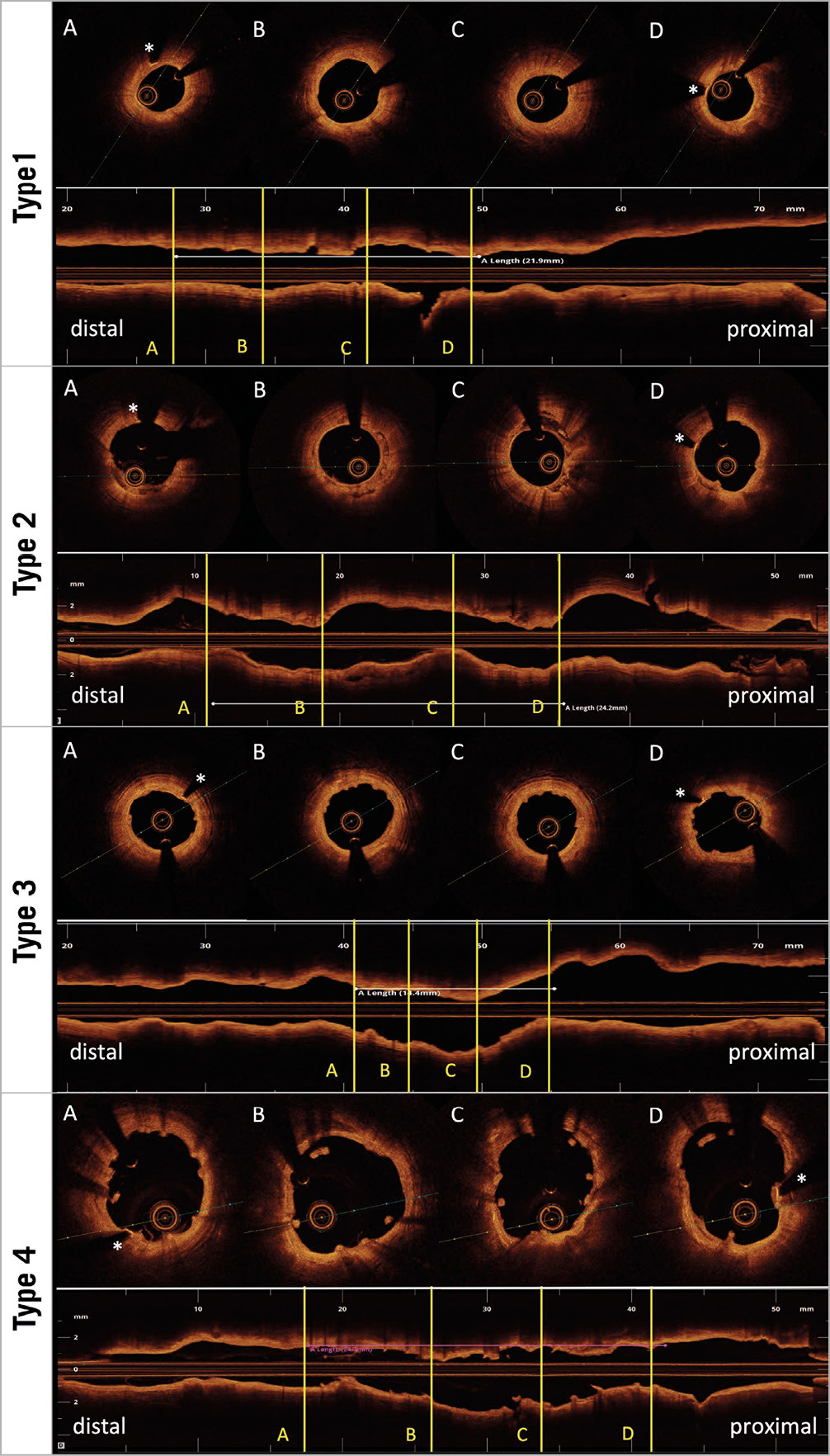
Figure 1. Healing and bioresorption patterns of magnesium-based bioresorbable scaffolds. Type 1: indiscernible struts. Type 2: visible struts completely integrated into the vessel wall. Type 3: visible struts protruding into the lumen causing a characteristic bumpy contour. Type 4: visible struts protruding and malapposed to the vessel wall. * radiopaque markers.
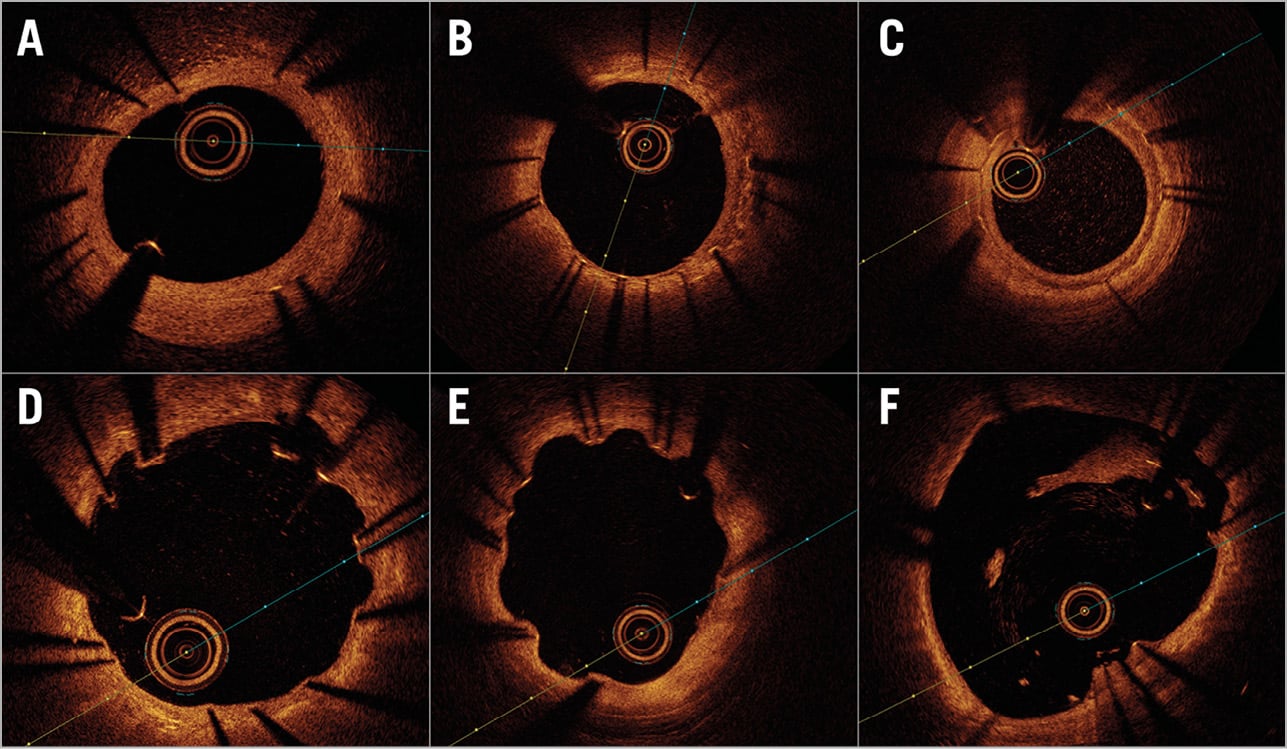
Figure 2. Qualitative OCT findings of permanent metallic sirolimus-eluting stents. A) Homogeneous neointima pattern. B) Heterogeneous neointima pattern. C) Layered neointima pattern. D) RUTTS ≥30% (ratio of uncovered to total stent struts). E) Coronary evagination. F) Incomplete stent apposition.
STATISTICAL ANALYSIS
Categorical variables are presented as counts and percentages, and continuous variables as mean±standard deviation (SD). Comparisons of categorical variables were estimated with the chi-square or Fisher’s exact test as appropriate. Comparisons of continuous variables between groups were estimated with the Student’s t-test. A two-sided p-value ≤0.05 was considered statistically significant. Statistical analysis was performed with SPSS software, version 20.0 (IBM Corp., Armonk, NY, USA).
Results
POPULATION
A total of 97 patients out of 108 included in the three OCT institutions underwent angiographic and OCT imaging at one year. According to the study flow chart (Figure 3), 2 patients died before the scheduled angiography, 8 patients refused angiographic follow-up and 2 patients presented with acute stent thrombosis who were treated with thrombus aspiration and balloon dilatation. One of the patients with stent thrombosis also underwent scheduled 12-month angiography. Two patients were excluded due to suboptimal image quality (n=1) and the incapacity of the OCT catheter to cross the stent segment resulting in incomplete stent imaging (n=1). Therefore, a total of 95 patients were included in the present study (MgBRS=48 and SES=47) and were analysed in the “OCT per protocol group”.
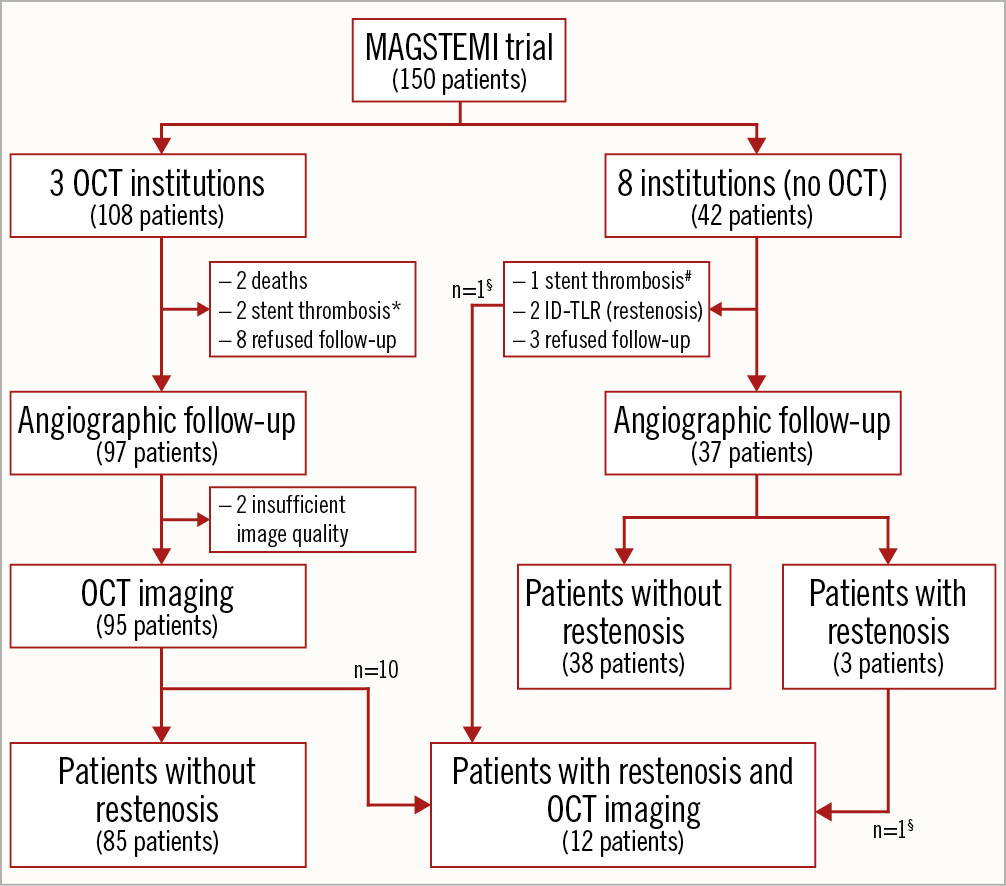
Figure 3. Study flow chart. * One patient with acute stent thrombosis underwent one-year angiographic and OCT follow-up. # One patient with acute stent thrombosis underwent one-year angiographic follow-up. § Two patients with restenosis underwent OCT imaging in a study institution with no OCT protocol. ID-TLR: ischaemia-driven target lesion revascularisation.
The MAGSTEMI trial had 14 patients with in-segment angiographic restenosis; all of them were included in the MgBRS arm6. Ten of these presented with angiographic restenosis and underwent OCT imaging as per protocol (in one of the three OCT institutions). These cases were already included in the “OCT per protocol group”. Moreover, two patients with in-segment restenosis included in two different institutions also underwent OCT imaging. Therefore, 12 out of 14 patients with MgBRS restenosis had OCT imaging. These 12 cases were analysed in the “OCT per restenosis group”.
CLINICAL AND PROCEDURAL CHARACTERISTICS
The clinical, procedural and angiographic characteristics of patients included in the OCT per protocol group (n=97) were similar to those observed in patients without OCT (n=37). These data are shown in Supplementary Table 1-Supplementary Table 3.
The clinical characteristics of all patients included in the OCT per protocol group are shown in Table 1. There were no statistically significant differences between the MgBRS and SES arms. Supplementary Table 4 shows the procedural characteristics of the OCT per protocol group. Procedural characteristics were similar between groups except for the percentage of post-dilatation, which was significantly higher in the MgBRS group (94.1% vs 16.7%; p=0.001).
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSIS
The QCA characteristics of the OCT per protocol group are shown in Table 2. Baseline post-PCI results showed significantly smaller in-device minimal lumen diameter (2.50±0.30 vs 2.71±0.40 mm; p=0.005) and greater residual diameter stenosis (11.05±5.75 vs 7.83±4.78%; p=0.024) with MgBRS than with SES. At one year, in-device late lumen loss was statistically significantly greater with MgBRS (0.56±0.47 mm) than with SES (0.06±0.25 mm), p=0.001.
OCT CHARACTERISTICS
Table 3 shows the qualitative OCT findings of the OCT per protocol group. The healing and bioresorption patterns between the devices were different in the two groups. Indiscernible struts were observed in 33.3% versus 0% of patients (p<0.001); visible struts completely integrated into the vessel wall were observed in 22.9% versus 63.8% (p<0.001); visible protruding struts were observed in 37.5% versus 31.9% (p=0.556); and visible protruding and malapposed struts were observed in 6.3% versus 4.3% (p=0.663), respectively. Figure 4 shows a case treated with MgBRS presenting with complete bioresorption of the device at one year. Scaffold discontinuities were observed in 10.4% and 0% of patients (p=0.023), respectively.
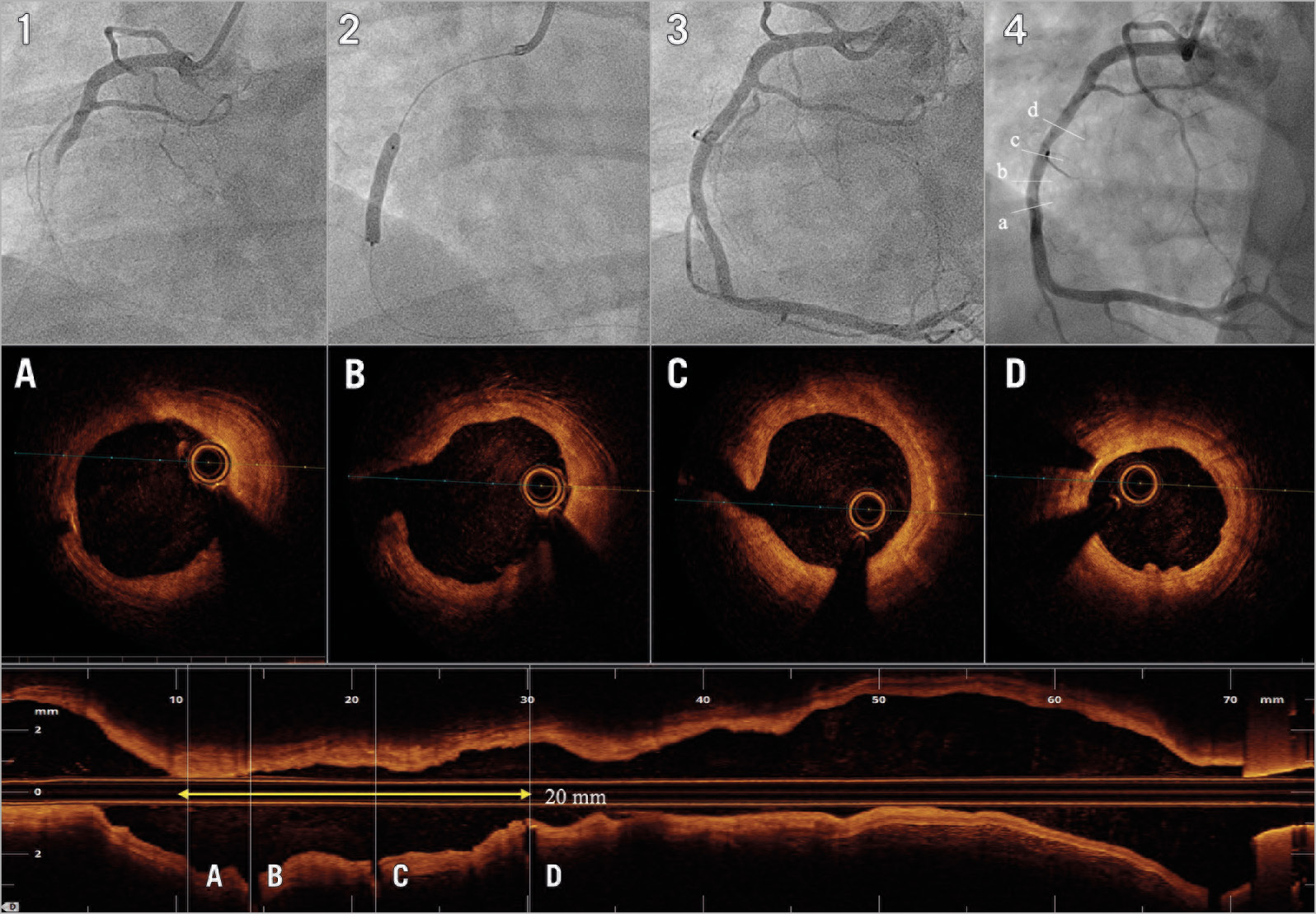
Figure 4. Patient treated with MgBRS with complete bioresorption at one year. A 43-year-old male presented with inferior STEMI. Image 1 shows acute occlusion of the right coronary artery. After thrombus aspiration and predilatation, the patient was treated with a 3.5×20 mm MgBRS (image 2). Image 3 shows the final result after post-dilatation with a 3.5 non-compliant balloon. At 12 months, the patient underwent coronary angiography (image 4). Images A, B, C and D depict the one-year OCT imaging showing most of the cross-sections with indiscernible struts.
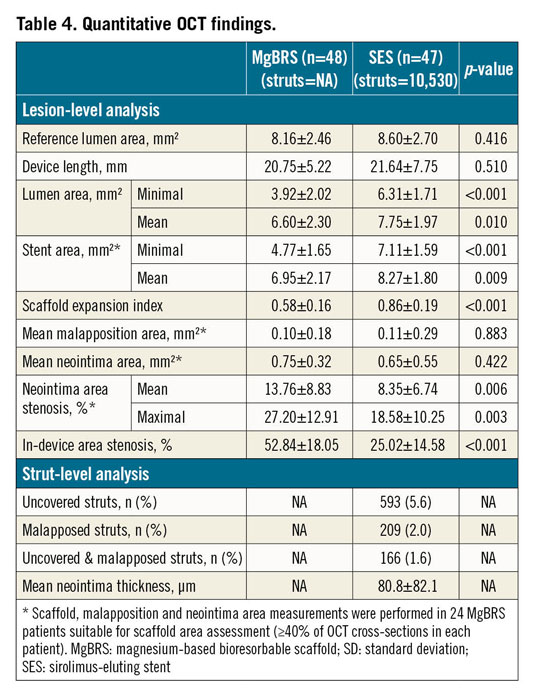
Quantitative OCT findings are shown in Table 4. Strut coverage of MgBRS was not suitable for quantitative analysis. SES presented with 5.6% of uncovered struts and 2.0% of malapposed struts. Minimal lumen area was smaller (3.92±2.02 vs 6.31±1.71 mm²; p<0.001) and area stenosis was greater (52.84±18.05 vs 25.02±14.58%; p<0.001) with MgBRS than with SES. Scaffold measurements were only feasible in 50% of the MgBRS population, with the expansion index of the device being smaller than with SES (0.58±0.16 vs 0.86±0.19; p<0.001).
DESCRIPTION OF CASES WITH IN-SEGMENT MgBRS RESTENOSIS
A total of 14 patients presented with in-segment MgBRS restenosis in the MAGSTEMI trial6. A summary of the 14 cases is described in Supplementary Table 5. Only one case with MgBRS required unscheduled coronary angiography due to angina symptoms at nine months. The other 13 cases were asymptomatic and were observed during the 12-month scheduled, per protocol, coronary angiography. Twelve out of 14 cases with MgBRS restenosis had OCT imaging and were included in the “OCT per restenosis group”. These cases are shown in Supplementary Figure 1-Supplementary Figure 12. According to the OCT qualitative analysis, scaffold collapse (n=5; 42%) was the main cause of scaffold failure. There were 3 cases (25%) with unknown causes due to the advanced bioresorption state of the scaffold at one year, and 2 cases (17%) were attributed to excessive neointima tissue (1 case [8%] had a combination of scaffold collapse and excessive neointima tissue and 1 case [8%] was attributed to scaffold discontinuity).
Discussion
The main findings of the present study are: 1) remnant struts were observed in 70% of patients treated with MgBRS at one year; 2) both MgBRS and SES exhibited a low degree of neointima since >35% of patients presented with protruding or protruding and malapposed struts as the most predominant healing patterns; 3) patients treated with MgBRS had smaller lumen dimensions and greater area stenosis than patients treated with SES at one-year follow-up; and 4) according to observations performed in 40% of OCT cross-sections with remnant scaffold struts, scaffold collapse seemed to be the main mechanism of scaffold restenosis.
The randomised MAGSTEMI study is the first head-to-head comparison of MgBRS versus the gold standard permanent metallic stent. The first generation of MgBRS (AMS®; Biotronik) had thick struts (165 μm) and no drug elution. The first-in-man investigation using this iteration reported 47.5% angiographic restenosis that was mainly attributed to scaffold recoil and excessive neointima response9. The second generation of the MgBRS scaffold (DREAMS®; Biotronik) was aimed at prolonging the radial force and inhibiting neointima formation by reducing the strut thickness (120 μm), coating the strut surface with bioresorbable polymer (poly lactic-co-glycolic acid) and coating the scaffold with an antiproliferative drug (paclitaxel). The first-in-man study using this iteration also showed a remarkable lumen loss (0.65±0.50 mm) and restenosis rate (17%) at six months. According to IVUS, lumen loss was mainly driven by scaffold recoil (73%)10. The third and current generation of MgBRS (DREAMS-2 or Magmaris; Biotronik) aimed to prevent scaffold recoil by enlarging the strut thickness (150 μm) and coating the scaffold with more stable polymer (poly-lactic acid). This iteration, coated with sirolimus, was evaluated in the first-in-man BIOSOLVE-II trial11. In this study, angiographic results were acceptable (lumen loss of 0.44±0.36 mm and 5% restenosis), and IVUS did not show relevant scaffold recoil at six months11.
However, an OCT substudy of the BIOSOLVE-II trial showed that patients treated with overexpanded devices (expansion index >1.0 at the end of the baseline procedure) presented with greater lumen loss than patients treated without overexpansion. The authors hypothesised that excessive overexpansion during implantation could provoke polymer fractures which could induce premature degradation of the magnesium alloy and an early loss of the radial force12. In the present study, patients presenting with greater lumen loss (>0.50 mm) at one year had a smaller baseline reference vessel diameter (RVD) (2.73 mm vs 2.92 mm; p=0.07) and were treated using a higher balloon to artery ratio (1.18 vs 1.12; p=0.08) than patients with less angiographic lumen loss (≤0.5 mm). Therefore, scaffold overexpansion was also probably related to in-scaffold restenosis.
On the other hand, the BIOSOLVE-II study included patients with stable coronary artery disease. In contrast, the MAGSTEMI trial only included patients with STEMI. It is plausible that inflamed coronary plaques, such as culprit STEMI plaques, may also speed the degradation of the scaffold polymer which could facilitate scaffold recoil. In a series of cases with clinically driven MgBRS restenosis, scaffold collapse was identified as the main cause of scaffold failure (58%), as assessed by OCT; five out of seven cases with scaffold collapse were implanted in the setting of STEMI13. In the present study, scaffold collapse was identified in 50% of cases with angiographic restenosis. However, there were also three (25%) patients with in-scaffold restenosis who presented with indiscernible struts, classified as unknown mechanism, in whom scaffold collapse cannot be discounted.
Scaffold failure mechanisms seem different between different types of bioresorbable scaffold (BRS). Scaffold discontinuities were attributed as being the main cause of scaffold failure using polymer-based BRS (Absorb™; Abbott Vascular)14. In event-free STEMI patients treated with polymeric BRS, strut discontinuities were observed in 26% at three years15. In the present study, only 10% of cases presented with scaffold discontinuities. Moreover, in a series of cases with MgBRS failure, only 33% were attributed to fractures; all of those cases presented as stable or unstable angina syndromes without angiographic signs of scaffold thrombosis13. This reinforces the hypothesis that MgBRS have lower thrombogenicity and may have a lower percentage of scaffold thrombosis than polymeric BRS, even in cases with strut discontinuities. In a swine arteriovenous shunt model including permanent SES, MgBRS and polymer-based BRS, MgBRS showed less platelet coverage (3.0%) than SES (4.6%) and polymer-based BRS (21.8%)16.
Limitations
The present study has several limitations. First, the bioresorption state of MgBRS, as assessed by OCT, hampers the quantification of some of the study findings. For example, scaffold collapse assessment has been based on only 40% of OCT cross-sections suitable for scaffold area measurement. It is uncertain if the other cases would have shown similar results. Second, the healing and bioresorption pattern is often heterogeneous showing contiguous regions with remnant and indiscernible struts. The qualitative patterns used in the present investigation represent the most common healing pattern throughout the scaffold. Third, due to the nature of the study, which included acute patients in the setting of PPCI procedures, there was no OCT imaging during the implantation. Serial assessment of OCT images between baseline and one year would allow better assessment of the healing pattern and scaffold recoil. Finally, the MAGSTEMI trial included <5% of all STEMI patients undergoing PPCI during the study recruitment; this is indicative of a selected group of patients not representative of the all-comer population.
Conclusions
At one year, both MgBRS and SES exhibited a low degree of neointima healing. However, MgBRS had smaller lumen dimensions and greater diameter stenosis than SES. Although the advanced bioresorption state of MgBRS hampers the assessment of scaffold dimensions, the analysis of patients with remnant scaffold struts indicated a low expansion index and suggests scaffold recoil as the most plausible cause of lumen loss. Moreover, scaffold collapse was observed in at least 50% of cases with angiographic restenosis. Future generations of MgBRS should increase the radial force and prolong the scaffolding time of the device.
|
Impact on daily practice The current generation of the magnesium-based bioresorbable scaffold (MgBRS) shows a high percentage of angiographic restenosis in STEMI patients. According to the present study, the most plausible explanation for this is scaffold collapse. New generations of MgBRS should increase and prolong the radial force of the device and test its efficacy in powered randomised trials with imaging substudies. |
Appendix. Study collaborators
Xavier Freixa, MD, PhD; Hospital Clínic i Provincial de Barcelona, IDIBAPS (Institut d’Investigacions Biomèdiques August Pi i Sunyer), Barcelona, Spain. Monica Masotti, MD, PhD; Hospital Clínic i Provincial de Barcelona, IDIBAPS (Institut d’Investigacions Biomèdiques August Pi i Sunyer), Barcelona, Spain. Jose Luís Ferreiro, MD; Hospital Universitari de Bellvitge, Institut d’Investigació Biomèdica de Bellvitge (IDIBELL), Universitat de Barcelona, L’Hospitalet de Llobregat, Spain. Gerard Roura, MD, PhD; Hospital Universitari de Bellvitge, Institut d’Investigació Biomèdica de Bellvitge (IDIBELL), Universitat de Barcelona, L’Hospitalet de Llobregat, Spain. Teresa Bastante, MD; Hospital Universtiario La Princesa, Instituto de Investigación Sanitaria-IP, Madrid, Spain. Marcelo Jiménez, MD; Hospital Universitari de Sant Pau, Barcelona, Spain. Imanol Otaegui, MD; Hospital Universitari Vall d’Hebrón, Barcelona, Spain. Pascual Bordes, MD; Hospital General de Alicante, Alicante, Spain. Andres Iñiguez, MD; Hospital Alvaro Cunqueiro, Vigo, Spain.
Funding
This work was supported by the Spanish Heart Foundation.
Conflict of interest statement
J. Gómez-Lara and L. Ortega-Paz have received fees from BARCICORE-lab. M. Sabaté is a consultant for Abbott Vascular and iVascular (not related to the present study). A. Cequier has received grants and personal fees from Abbott Vascular, Medtronic, Boston Scientific, and Biosensors (not related to the present study). P. Salinas has received speaker fees from Terumo, Boston Scientific, AlviMedica and Biomenco (not related to the present study). S. Brugaletta is a consultant for Boston Scientific and iVascular and has received a research grant to his institution from AstraZeneca (not related to the present study). A. Regueiro has received speaker fees from Abbott and Cardinal Health (not related to the present study). The other authors/study collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

