
Drug-eluting stent implantation in the context of ST-elevation myocardial infarction (STEMI) has been associated with delayed strut coverage related to subjacent plaque inflammation and late acquired malapposition in relation to resorption of the thrombus trapped between the stent and the vessel wall. A higher risk of stent thrombosis has been described in this population. In this context, bioresorbable scaffolds (BRS) offer potential advantages given their disappearance from the vessel wall once their scaffolding and drug-eluting function has been completed. Furthermore, STEMI patients are a subgroup well suited to BRS implantation given that they are generally younger and with softer target plaques for stenting that allow proper expansion of the scaffolds (an aspect that has been shown to be key to their performance).
Previous studies using polymeric BRS had shown promising results in STEMI patients1,2. However, the higher risk of thrombosis, systematically demonstrated in multiple trials with these devices, has precluded their introduction in clinical practice. Magnesium-based BRS (MgBRS) have evolved from their original design with changes aiming to improve radial force and resorption time in order to decrease the high risk of restenosis observed with the initial iterations. MAGSTEMI was a randomised trial designed to evaluate the value of MgBRS (DREAMS-2 or Magmaris®; Biotronik, Bülach, Switzerland) in patients with STEMI. The study demonstrated a larger vasodilatory response to nitroglycerine (NTG) at one year in patients treated with MgBRS as compared with those treated with sirolimus-eluting stents (SES). However, the late lumen loss and rate of restenosis at one-year follow-up were higher in the MgBRS group3 (Table 1).
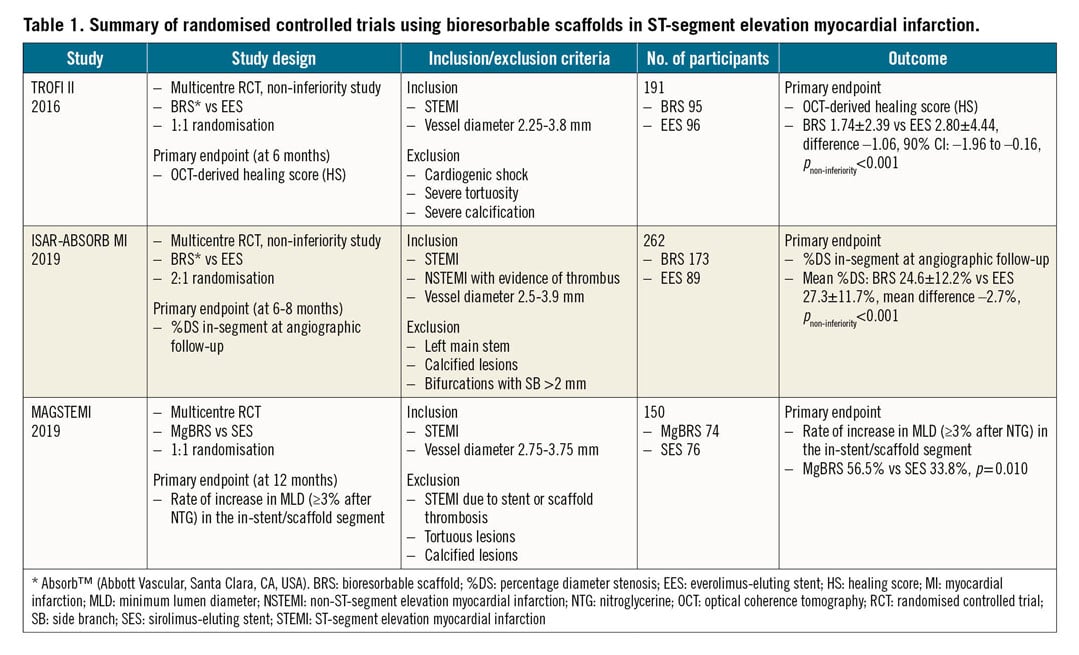
In this issue of EuroIntervention, Gomez-Lara et al4 report the results of the MAGSTEMI OCT substudy in which imaging with OCT was performed at one-year follow-up in a subgroup of patients with the objective of evaluating vascular healing and causes of restenosis.
They demonstrate a high proportion of visible MgBRS struts at one year with a predominant pattern of protruding struts in both the scaffold and the SES. The MgBRS showed smaller lumen dimensions, mainly related to a decrease in scaffold size as no differences in neointimal area were shown. Further, scaffold collapse is reported as the main mechanism of restenosis.
A previous report of the BIOSOLVE-II trial reported that MgBRS struts were visible by OCT in 93.3% of the frames at one year and scaffold areas were traceable in 78.8% of frames. The present study, however, demonstrates around 67% of remanent struts by OCT in the MgBRS population. Furthermore, scaffold area was clearly visible in only 40% of OCT cross-sections at one year5. This discordance could be related to differences in resorption depending on the substrate (the BIOSOLVE-II trial included only stable or unstable angina patients as compared with MAGSTEMI), and to different definitions used to determine the presence of struts.
Regarding healing, these results are encouraging, showing an adequate integration of the scaffold in the vessel wall with no signs of persistently uncovered struts or late acquired malapposition (as has been described for polymeric BRS). STEMI is a complex scenario for vessel healing given the frequent presence of underlying plaque rupture and inflamed necrotic core that can delay strut coverage by functional endothelium. In this regard, the majority of MgBRS struts were either indiscernible, integrated or protruding, but covered by tissue as assessed by OCT. The functional results of the main MAGSTEMI trial, showing a better vasodilatory response to NTG in the MgBRS group, reinforce the idea of a favourable vascular healing with this device.
The study also provides important insights into the causes of a larger late lumen loss in MgBRS as compared with SES observed in the MAGSTEMI trial. This is mainly attributed to scaffold recoil at follow-up and not to excessive neointimal growth. These results are in line with the serial intravascular ultrasound (IVUS) imaging results of the BIOSOLVE-II study showing a progressive decrease in the minimum scaffold area of the MgBRS between post implantation and 12-month follow-up without a significant increase in neointimal hyperplasia6. Another analysis of BIOSOLVE-II including 70 patients with OCT imaging at baseline and 6- to 12-month follow-up demonstrated that late scaffold recoil was the major contributor to lumen area decrease at follow-up5. Scaffold collapse has been described in previous cases of MgBRS restenosis; the current study reinforces its significant role in late scaffold failure7.
The definition of scaffold recoil and collapse in the present study is however limited by the lack of OCT imaging after scaffold implantation. The acute angiographic results demonstrate a smaller minimum luminal diameter after the procedure with MgBRS as compared with SES, even though post-dilatation was performed more commonly in patients in the MgBRS arm. It is therefore possible that part of the underexpansion observed at follow-up and considered as “late recoil” was actually caused by an inadequate procedural result. Further, in the present report, scaffold area measurements were only possible in 40% of the cross-sections given the advanced resorption process in most devices.
Acknowledging these limitations, these findings are relevant for future developments in the MgBRS field. Scaffold recoil is identified as the main mechanism for late lumen loss, suggesting the need to refine the resorption period to provide adequate vessel scaffolding for an appropriate duration. Successive iterations of the device have changed the strut thickness and the drug elution in order to decrease restenosis, but the formula to obtain enough radial force during the required period seems to need further refinements, especially if the device is planned for use in more challenging scenarios that require good scaffold support (i.e., calcification).
The influence of the underlying substrate in the speed of resorption is another aspect that requires further investigation and can explain the greater restenosis rate observed in MgBRS implanted in the context of STEMI. Data are scarce but, in a previous case series of MgBRS failure, scaffold collapse was observed predominantly in patients originally treated in the context of STEMI7. These data are somehow conflicting with the results of the OCT substudy of BIOSOLVE-II that reported a relation between late scaffold recoil and plaque type (with lipid plaques being associated with less scaffold recoil and fibrous plaques with larger scaffold shrinkage)5. In this context, if scaffold recoil is the main mechanism of late luminal decrease, it is difficult to understand the greater late lumen loss and percentage of restenosis observed in MAGSTEMI as compared with BIOSOLVE-II when most probably the proportion of lipid plaques in MAGSTEMI was much higher than that in the stable patients included in BIOSOLVE-II. Other factors influencing the speed of resorption and loss of radial force in the context of STEMI not purely related to plaque stiffness might explain these results.
In the MAGSTEMI trial there was only one case of acute stent thrombosis and no late thrombosis occurred. The OCT analysis at follow-up reveals that discontinuities (identified as one of the main causes of stent thrombosis in polymeric scaffolds) were infrequent with MgBRS. Furthermore, when they were present, they did not induce thrombosis, pointing towards a thromboresistance of MgBRS, as observed in bench studies8.
In conclusion, the current study provides new insights into MgBRS performance in STEMI, one of the more attractive scenarios for the use of bioresorbable scaffolds. These findings provide explanations for the causes of MgBRS failure in this context and can guide future improvements aimed to making these devices competitive with current-generation DES in patients in whom a bioresorbable device could be considered beneficial.
Conflict of interest statement
N. Gonzalo reports being a speaker at educational events for Abbott and Boston Scientific. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

