
Introduction
Bioresorbable scaffolds (BRS) were introduced into clinical practice to overcome long-term limitations of drug-eluting stents (DES), but they were instead associated with a high rate of target lesion revascularisation (TLR) and thrombosis. Whereas scaffold discontinuity was the most frequent mechanism for polymeric BRS TLR1, there are no studies on the causes of second-generation drug-eluting absorbable metal scaffold (MgBRS) TLR (Magmaris; Biotronik, Bülach, Switzerland). We sought to determine the optical coherence tomography (OCT) findings in patients who experienced an MgBRS TLR. Moreover, we performed a systematic review of the reported cases.
Methods
A retrospective screening was conducted to identify all the patients with an MgBRS TLR documented by OCT at six tertiary hospitals in Spain. Clinical data were anonymously collected with the approval of the local ethics committees. The patient selection process is shown in Supplementary Appendix 1. OCT and quantitative coronary angiography data were analysed offline in a core lab (BARCICORE-lab, Barcelona, Spain) using dedicated software (LightLab Imaging, Mountain View, CA, USA, and CAAS; Pie Medical Imaging, Maastricht, the Netherlands). A qualitative assessment of the entire OCT pullback was performed by two investigators (L. Ortega-Paz and S. Brugaletta) to determine the main OCT findings associated with TLR. These were predefined as scaffold discontinuity, malapposition, evagination, uncovered struts, neoatherosclerosis, restenosis without neoatherosclerosis, scaffold underexpansion/device collapse, edge dissection, or edge-related disease progression1. In case of disagreement, a third analyst (J. Gomez-Lara) was asked in order to reach a consensus. The complete OCT definitions are detailed in Supplementary Appendix 2.
CASE REPORTS FROM THE LITERATURE
Two independent reviewers (L. Ortega-Paz and S. Brugaletta) performed a systematic review of the literature applying the methodology detailed in Supplementary Appendix 3 and Supplementary Figure 1.
Results
Between December 2016 and October 2018, 100 patients received MgBRS at the participating institutions. No deaths were reported, and 12 cases of TLR (12%) were found (Table 1). OCT data were available in all the TLR cases. The patients were mainly male (92%) with a median age of 56 (49-61) years. At the index procedure, all patients received one scaffold with a median diameter of 3.5 (3.0-3.5) mm and length of 20 (15-25) mm. In 10 out of 12 patients the device was implanted in an off-label scenario, mainly ST-segment elevation myocardial infarction (STEMI). Regarding the predilation, sizing, and post-dilation (PSP) technique, in one patient predilation was not performed; post-dilation was carried out in eight patients but in seven of them with a maximal balloon-to-scaffold ratio of 1:1. The complete patient and procedural characteristics are shown in Supplementary Table 1. There were no significant differences between the included and not included patients.
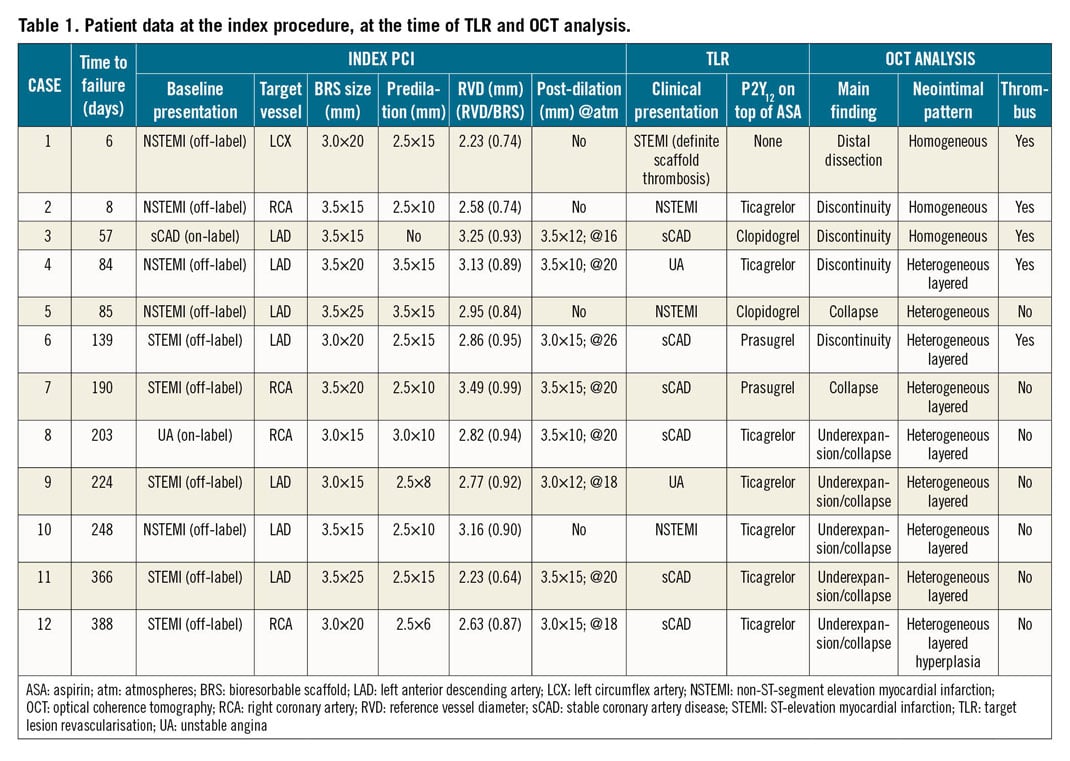
At the time of the TLR, six (50%) patients experienced an acute coronary syndrome (ACS), with one case of subacute definite scaffold thrombosis, due to abrupt interruption of dual antiplatelet treatment (DAPT), four days after the index procedure. The median occurrence of TLR was 164 days (IQR 63-242).
The main OCT findings were device underexpansion/collapse (n=7; 58%), scaffold discontinuity (n=4; 33%), and distal edge dissection (n=1; 9%) (Figure 1, Supplementary Figure 2, Supplementary Figure 3, Moving image 1-Moving image 3). Two cases of underexpansion (#5 and #7 in Table 1) also had OCT data at the index PCI, showing a well apposed and expanded scaffold, therefore confirming the occurrence of a device collapse. No other baseline OCT data were available. The scaffold discontinuity phenomenon was identified at an earlier stage than underexpansion/collapse (70 days [32-11] vs 224 days [190-366]), p=0.022).
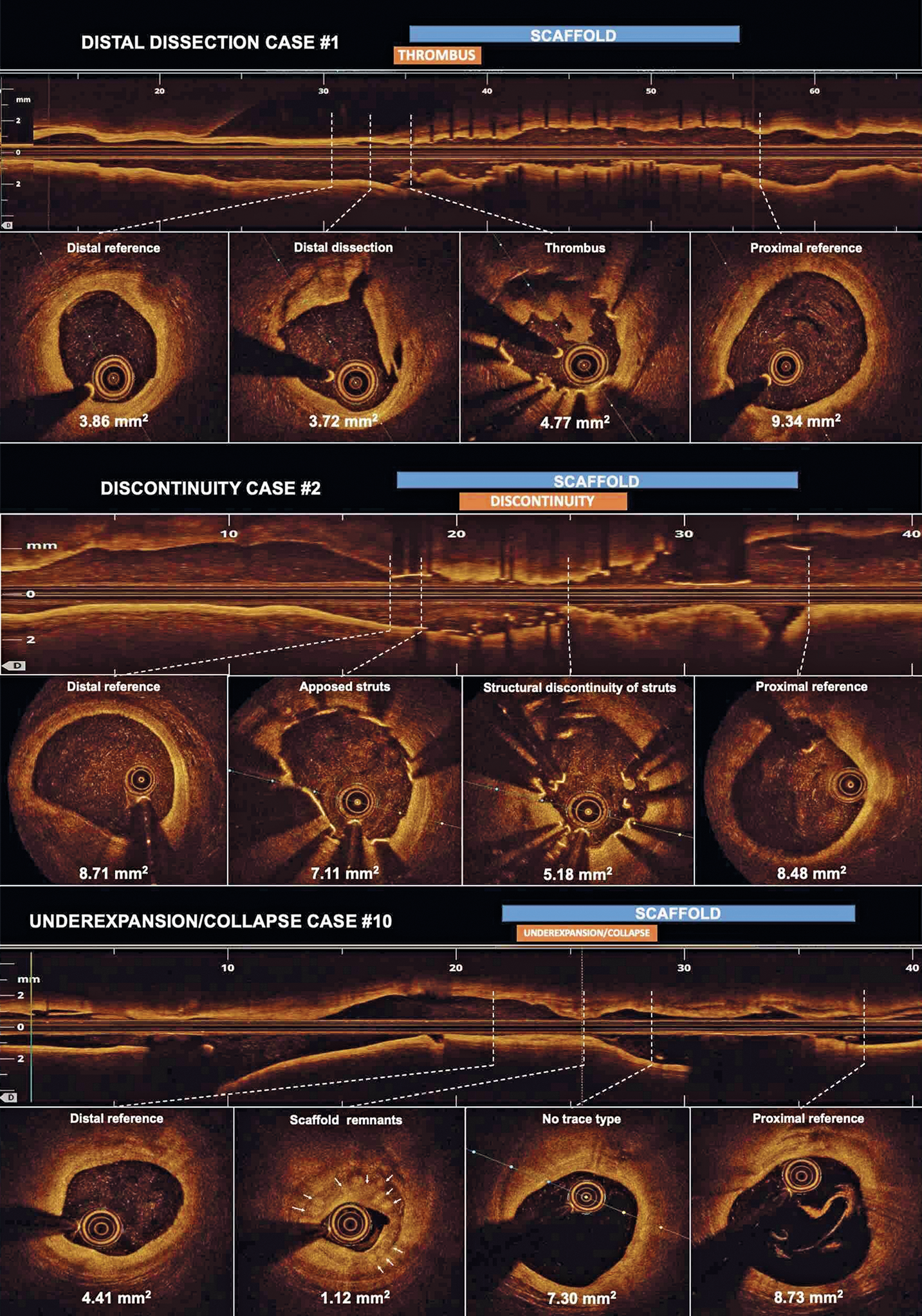
Figure 1. Second-generation drug-eluting absorbable metal scaffold target lesion revascularisation OCT findings. Minimum lumen area is shown per each cross-section. In case #2, predilation was performed before OCT assessment. Therefore, it cannot be ruled out that the scaffold structure was iatrogenically altered. A three-dimensional reconstruction of case #2 is shown in Supplementary Figure 3.
CASE REPORTS FROM THE LITERATURE
In the literature review, up to April 2019, we found six cases of MgBRS TLR with intravascular imaging assessment (Supplementary Table 2),2,3,4,5,6,7. The data relating to these cases are shown in Supplementary Appendix 4.
Discussion
The main findings of this case series study are the following. 1) The most frequent OCT findings were device underexpansion/collapse and scaffold discontinuity. 2) Scaffold discontinuity was found at an earlier stage than underexpansion/collapse. 3) The majority of the cases were off-label indications with a suboptimal PSP technique. 4) Only one case of scaffold thrombosis was observed.
In this retrospective analysis of patients treated with MgBRS who experienced TLR, the clinical characteristics and OCT findings resembled very closely those of the cases reported in the literature. Although both populations are very selected, the TLRs analysed may be related either to scaffold use in an off-label scenario or to a suboptimal PSP implantation technique8,9. Nevertheless, a recent study showed that the procedural characteristics do not impact on the MgBRS healing process10. It is worth mentioning that in first-in-man studies MgBRS were used in a very selected population, including only simple lesions and excluding ACS patients9. Notably, the off-label implantation in STEMI patients may be related to the high TLR rate because of the difficulties of accurate device sizing and worse clinical outcomes.
Of note is that, irrespective of adverse OCT findings, only one patient had definite scaffold thrombosis, in the context of early DAPT interruption, which is in line with previous studies showing a lower acute MgBRS thrombogenicity as compared to polymeric BRS11.
Eventually, prolongation of the scaffolding time and increasing the radial force could reduce the incidence of scaffold discontinuity or device collapse during follow-up. These hypotheses need to be confirmed in larger studies and should be taken into consideration for further improvements in the technology.
Limitations
This study has limitations that should be acknowledged. First, this is a retrospective study without a control group. However, to date, this is the largest cohort of MgBRS TLR analysed at a core lab facility. Second, only two cases had baseline and TLR OCT data. Third, for the assessment of MgBRS, the use of intravascular ultrasound (IVUS) and virtual histology may be better than OCT9, although the spatial resolution of OCT is higher than that of IVUS. Fourth, as the magnesium scaffold shadow disappears by six months, the struts can be ambiguously identified, making the discrimination of the OCT findings challenging. Fifth, the OCT definitions applied were derived from polymeric BRS studies. However, currently there are no standardised definitions for MgBRS failure assessment.
Conclusion
In patients who experienced an MgBRS TLR, the most frequent OCT finding was device underexpansion/collapse followed by scaffold discontinuity.
|
Impact on daily practice The most common OCT findings in patients who experienced an MgBRS TLR were device underexpansion/collapse and scaffold discontinuity. The discontinuity cases occurred at an earlier stage compared to device underexpansion/collapse. However, a scaffold thrombosis conditioning a myocardial infarction was unusual. All these data should be considered for the clinical decision-making process and further improvements in the technology. |
>Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Discontinuity case #2.
Underexpansion case #10.
Distal dissection case #1

