Abstract
Background: The long-term safety and performance of magnesium-based bioresorbable scaffolds (MgBRS) in ST-segment-elevation myocardial infarction (STEMI) patients are uncertain.
Aims: The aim of this study was to report the 3-year clinical outcomes of the MAGSTEMI trial.
Methods: This investigator-driven, multicentre, randomised, single-blind, controlled trial randomised STEMI patients 1:1 to MgBRS or to permanent metallic sirolimus-eluting stents (SES) at 11 academic centres. The main secondary endpoints included device-oriented composite endpoints (DoCE) and patient-oriented composite endpoints (PoCE), their individual components, any bleeding, and device thrombosis rate. All endpoints were defined according to the Academic Research Consortium. Events were adjudicated by an independent committee.
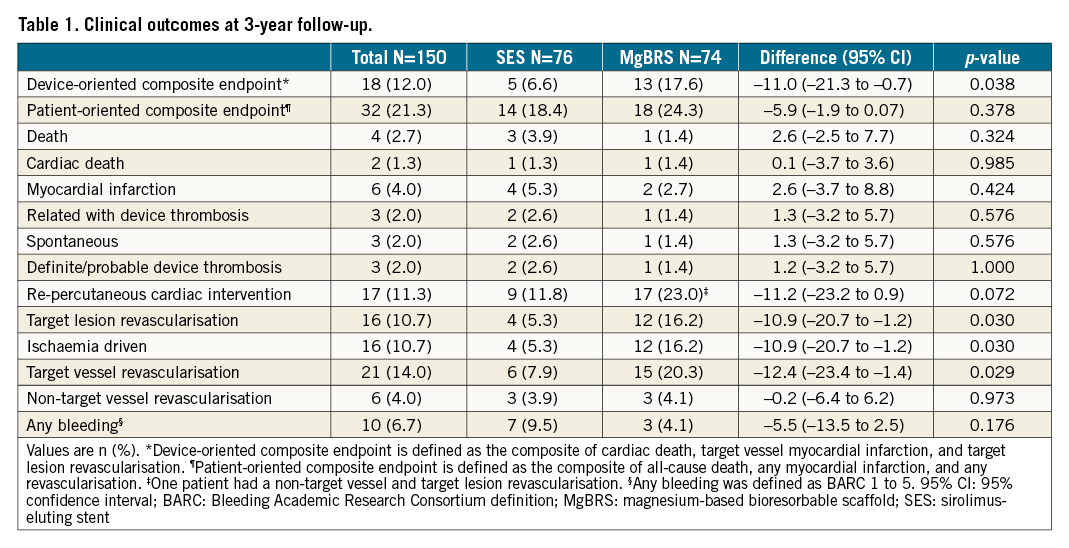
Results: Three-year clinical follow-up was obtained in 142 (90.0%) patients. At 3-year follow-up, MgBRS were associated with a higher rate of DoCE than SES (13 [17.6%] vs 5 [6.6%], diff −11.0 [95% CI: −21.3 to −0.7]; p=0.038). This difference was driven by an increased incidence of DoCE within the first year of follow-up. In the landmark analysis, there was no difference between 1 and 3 years (0 [0.0%] vs 1 [1.4%]; p=1.000). The difference in the rate of DoCE was driven by a higher incidence of target lesion revascularisation (TLR) in the MgBRS group compared to SES (12 [16.2%] vs 4 [5.3%]; diff −10.9% [95% CI: −20.7 to −1.2]; p=0.030). The difference in TLR was observed during the first year, with no further differences observed between 1 and 3 years (0 [0.0%] vs 1 [1.4%]; p=1.000).
Conclusions: At 3-year follow-up, MgBRS were associated with a higher rate of TLR, which was clustered within the first year, compared to SES.
Introduction
Patients treated with permanent metallic coronary stents may have an increased risk of device-related adverse events within the first 5 years of implantation1. The concept of a completely bioresorbable scaffold (BRS) that provides a temporary scaffolding was proposed as an innovative solution to overcome these device-related events. Two types of BRS have been studied in randomised clinical trials: polymeric BRS (typically polylactide-based), which have been withdrawn from daily clinical practice due to safety concerns, and magnesium-based BRS (MgBRS).
The MAGSTEMI trial (ClinicalTrials.gov: NCT03234348) was the first randomised clinical trial comparing the performance of MgBRS versus permanent metallic sirolimus-eluting stents (SES) in patients with ST-segment elevation myocardial infarction (STEMI)2. At 1-year follow-up, MgBRS were superior to SES in terms of vasomotor response capacity to pharmacological agents. However, MgBRS were associated with lower angiographic efficacy and a higher rate of target lesion revascularisation (TLR) without thrombotic safety concerns.
In the prespecified optical coherence tomography (OCT) substudy of the MAGSTEMI trial, at 1 year, both MgBRS and SES exhibited a low degree of neointima healing. Still, lumen dimensions were smaller with MgBRS, assessed by means of OCT3. Although the advanced bioresorption state of MgBRS limited the evaluation, the scaffold collapse seemed to be the main mechanism of restenosis. Moreover, an OCT report has found MgBRS scaffold remnants present at 2.5-year follow-up which are potentially related to adverse events4.
With polymeric BRS, dual antiplatelet therapy (DAPT) during the first year after implantation was associated with a reduction in adverse events, without a clear benefit beyond 1-year follow-up5. It should be noted that there are no randomised data of the safety and performance of MgBRS in STEMI patients after 1-year follow-up, the timepoint at which DAPT is usually discontinued in STEMI patients.
We herein report the 3-year clinical outcomes of the MAGSTEMI trial beyond the 1 year of DAPT. Furthermore, we aim to correlate the 1-year OCT vascular healing findings with the 3-year clinical outcomes.
Methods
Study design and oversight
The MAGSTEMI study was an investigator-initiated, multicentre, prospective and randomised clinical trial. This work was supported by the Spanish Heart Foundation. The complete trial design was reported previously6. Briefly, a total of 150 STEMI patients were randomised 1:1 to MgBRS or to SES in 11 academic hospitals. All patients were requested to undergo angiographic and vasomotor examination with nitroglycerine at 1-year follow-up. The study was approved by the Hospital Clinic of Barcelona institutional review board (IRB) and by the local IRBs of all the participating centres. All the patients provided written informed consent. The study was conducted in compliance with the study protocol, the Declaration of Helsinki, BS EN ISO 14155 Part 1 and Part 2, and applicable local requirements. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study population
We enrolled patients with STEMI who were undergoing primary percutaneous coronary intervention (PCI) and had one target lesion that was considered to be suitable for either MgBRS or SES implantation. All inclusion and exclusion criteria were reported elsewhere6.
Optical coherence tomography
At 1-year follow-up, OCT imaging was performed after vasomotor examination with the Dragonfly OPTIS catheter (Abbott Vascular) according to standard procedures. OCT analysis was performed by a dedicated core laboratory (BARCICORE-lab, Barcelona, Spain) using specific software for analysis (LightLab Imaging; Abbott Vascular). The analysed segment included the stent region and the stent margins defined as the vessel segment 5 mm proximal and distal to the stent. One-year results were previously reported3. One-year quantitative OCT analysis results were previously reported3. Moreover, consensus on 1-year qualitative OCT analysis was agreed by 2 analysts (J. Gomez-Lara and L. Ortega-Paz). Scaffold discontinuity was defined as struts overhanging each other at the same angular sector, with or without malapposition, or isolated struts at the luminal centre without an obvious connection to other surrounding struts7. One-year qualitative OCT analysis results were previously reported3.
Follow-up
Patients will be followed up to 5 years after the index procedure. In this report, patients who completed 3-year follow-up were included in the analysis. The follow-up includes clinical visits or telephone contacts regarding cardiovascular drug use, hospitalisations, invasive or non-invasive diagnostic tests, and clinical events at 30 days, 6 months, 12 months, and annually up to 5 years.
Study endpoints
The primary endpoint was the in-stent/in-scaffold vasomotor response after nitroglycerine administration at 1 year (previously reported)2. MgBRS demonstrated a better vasomotor response than SES but evidenced a higher rate of TLR that led to an increased rate of device-oriented clinical endpoints (DoCE)2. Secondary endpoints were DoCE (composite of cardiac death, target vessel myocardial infarction, and ischaemia-driven target lesion revascularisation), patient-oriented composite endpoints (PoCE; composite of all-cause death, any myocardial infarction, and any revascularisation), their individual components, and stent/scaffold thrombosis, according to the Academic Research Consortium definition8. Bleeding events were defined according to the Bleeding Academic Research Consortium definition (BARC)9. Any bleeding was defined as BARC 1 to 5. An independent clinical events committee adjudicated adverse events. Revascularisation was considered ischaemia-driven if associated with any of the following: a non-invasive positive functional ischaemia study or an invasive positive ischaemia study; ischaemic symptoms and an angiographic minimal lumen diameter stenosis ≥50% by online quantitative coronary angiography (QCA); or, a diameter stenosis ≥70% by online QCA. All clinical endpoints were analysed by the “intention-to-treat” principle.
Statistical analysis
The clinical variables at 3 years are presented as incidence (95% confidence interval [CI], using the Wald confidence interval) and were compared with the χ2 or Fisher’s exact test. For time-to-event variables, time-to-failure curves were constructed using Kaplan–Meier estimates, and log-rank test results are displayed. A two-tailed p-value <0.05 was considered significant. The SAS v9.4 software was used for all analyses.
Data sharing
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Results
The complete 3-year clinical follow-up was obtained in 142 (90.0%) patients. Following current guidelines, DAPT was withdrawn at 12 months unless clinically indicated (i.e., patients with repeat revascularisation within the first year). Consequently, at 3 years, 13 (9.6%) patients remained on DAPT, 10 (14.5%) in the MgBRS group versus 3 (4.5%) in the SES group (p=0.051). Supplementary Figure 1 shows the yearly DAPT prescription throughout the trial. Overall, up to 2-year follow-up, DAPT prolongation was related to a higher rate of revascularisation in patients treated with MgBRS when compared to SES (11 [15.9%] vs 5 [6.6%]; p=0.087). Meanwhile, at 3-year follow-up, the main factor in the decision to prolong DAPT was the treating physician’s recommendation (9 [13.0%] vs 3 [4.5%]; p=0.064).
Clinical outcomes
Overall, at 3 years, MgBRS was associated with a higher rate of DoCE than SES (13 [17.6%] vs 5 [6.6%], diff −11.0% [95% CI: −21.3 to −0.7]; p=0.038). This difference was driven by an increased incidence of DoCE within the first year of follow-up (Figure 1). In the landmark analysis, there was no difference between 1 and 3 years (0 [0.0%] vs 1 [1.4%]; p=1.000) (Figure 2). The difference in the rate of DoCE was driven by a higher incidence of TLR in the MgBRS group compared to SES (12 [16.2%] vs 4 [5.3%]; diff −10.9% [95% CI: −20.7 to −1.2]; p=0.030) (Figure 2). Again, the difference in TLR was observed during the first year, with no further differences observed between 1 and 3 years (0 [0.0%] vs 1 [1.4%]; p=1.000) (Figure 2).
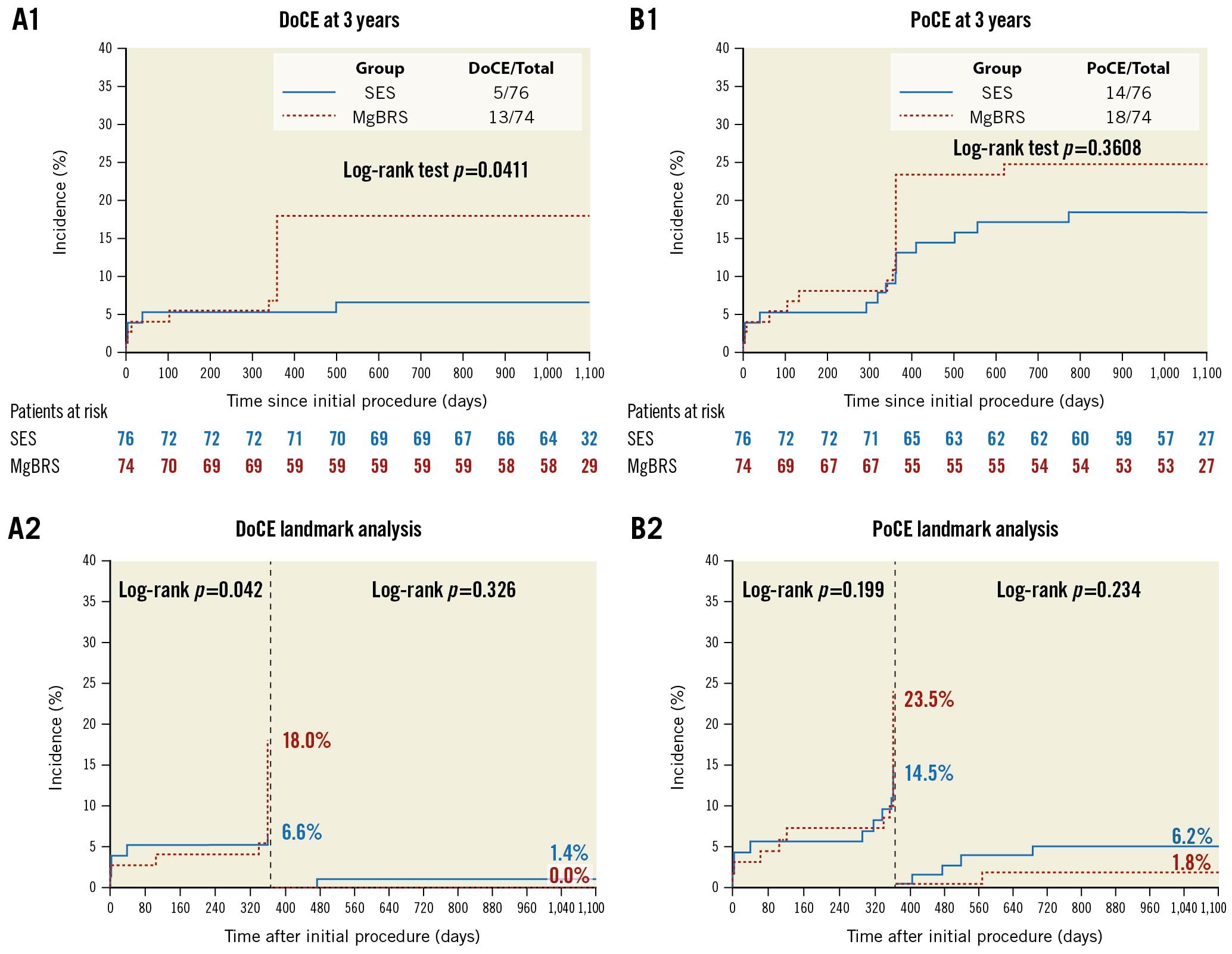
Figure 1. Time to failure curves of DoCE and PoCE between MgBRS vs SES in patients with STEMI. Device-oriented composite endpoint (DoCE) includes cardiac death, target vessel myocardial infarction, and target lesion revascularisation. Patient-oriented composite endpoint (PoCE) includes all-cause death, any myocardial infarction, and any revascularisation. MgBRS: magnesium-based resorbable scaffold; SES: permanent metallic sirolimus-eluting stent; STEMI: ST-segment elevation myocardial infarction
Rates of cardiac death were similar between MgBRS and SES (1 [1.4%] vs 1 [1.3%]; diff 0.1% [95% CI: −3.7 to 3.6]; p=0.985). In the landmark analysis, there were no cardiac deaths between 1- and 3-year follow-up. Similarly, rates of myocardial infarction were comparable at 3 years (2 [2.8%] vs 4 [5.3%]; diff 2.6% [95% CI: −3.7 to 8.8]; p=0.424) (Figure 2). In the landmark analysis, there was no difference between 1 and 3 years (1 [1.4%] vs 1 [1.4%]; p=1.000) . Overall, the combined patient-oriented clinical endpoint was similar between groups at 3 years (18 [24.3%] vs 14 [18.4%]; diff −5.9% [95% CI: −1.9 to 0.07]; p=0.378) (Figure 1). In the landmark analysis, there was no difference between 1 and 3 years (1 [1.4%] vs 4 [6.2%]; p=0.234). Furthermore, at 3 years, there were no differences in the rates of definite/probable device thrombosis (1 [1.4%] vs 2 [2.6%]; p=1.000) . Beyond 1 year, no instances of device thrombosis were observed in either group. Ultimately, at 3 years, there were no differences in the rates of any bleeding (7 [9.5%] vs 3 [3.9%]; diff −5.5 [95% CI: −13.5 to 2.5]; p=0.176) (Table 1). In the landmark analysis, there were no differences in any bleeding between 1 and 3 years (3 [3.9%] vs 1 [1.3%]; p=0.294) (Supplementary Table 1). In patients who experienced a bleeding event between 1 and 3 years, only one of them was on DAPT (aspirin and ticagrelor) (Supplementary Table 2).
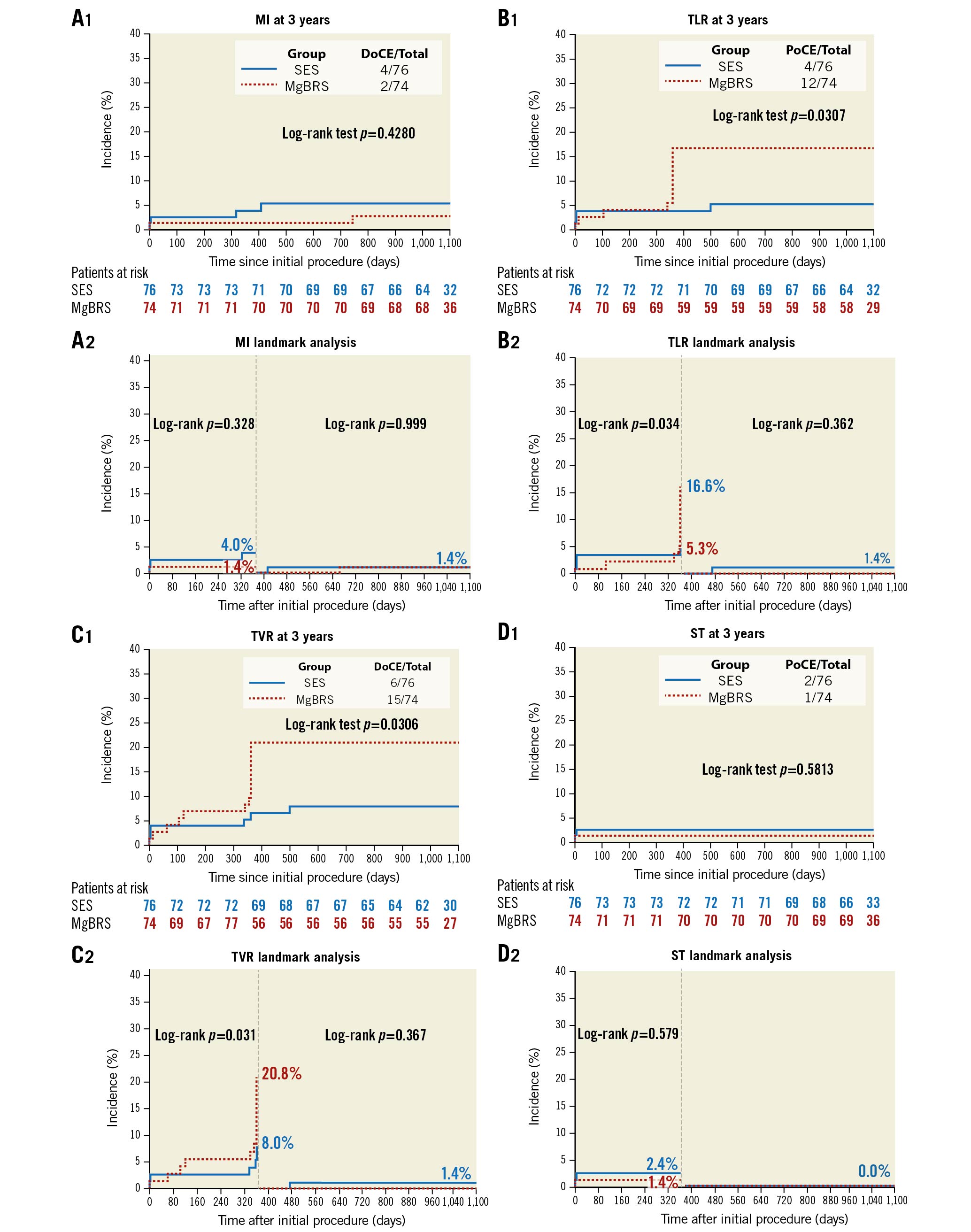
Figure 2. Time to failure curves of MI, TLR, TVR, and device thrombosis between MgBRS vs SES in patients with STEMI. Individual outcomes were defined according to the Academic Research Consortium definition. DoCE: device-oriented composite endpoint; MgBRS: magnesium-based resorbable scaffold; MI: myocardial infarction; PoCE: patient-oriented composite endpoint; SES: permanent metallic sirolimus-eluting stent; STEMI: ST-segment elevation myocardial infarction. TLR: target lesion revascularisation; TVR: target vessel revascularisation
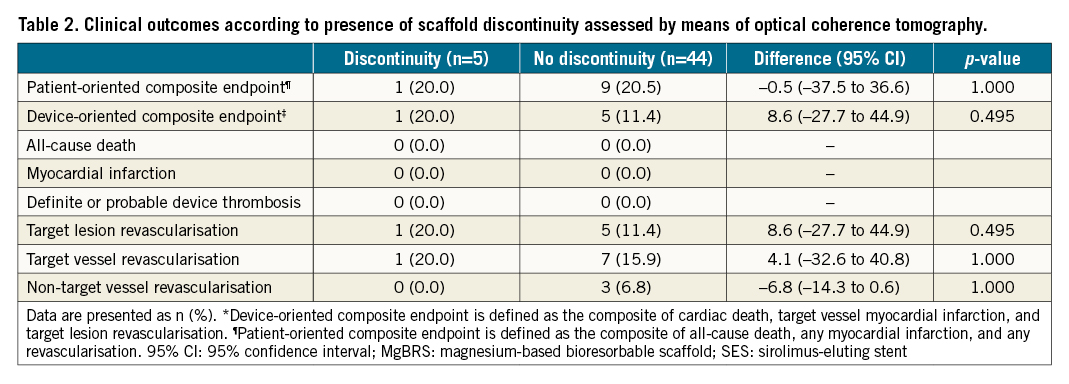
1-YEAR SCAFFOLD DISCONTINUITY AND 3-YEAR CLINICAL OUTCOMES IN MgBRS
We did not find any association between the presence of scaffold discontinuities at 1 year assessed by means of OCT and clinical outcomes at 3-year follow-up (Table 2).
Discussion
Our main findings are 1) at 3-year follow-up, MgBRS were associated with a higher rate of DoCE compared to SES; 2) 1-year scaffold discontinuity OCT findings were not associated with 3-year clinical outcomes, 3) at 3-year follow-up, patients treated with MgBRS were more frequently on DAPT compared to those treated with SES (Central illustration).
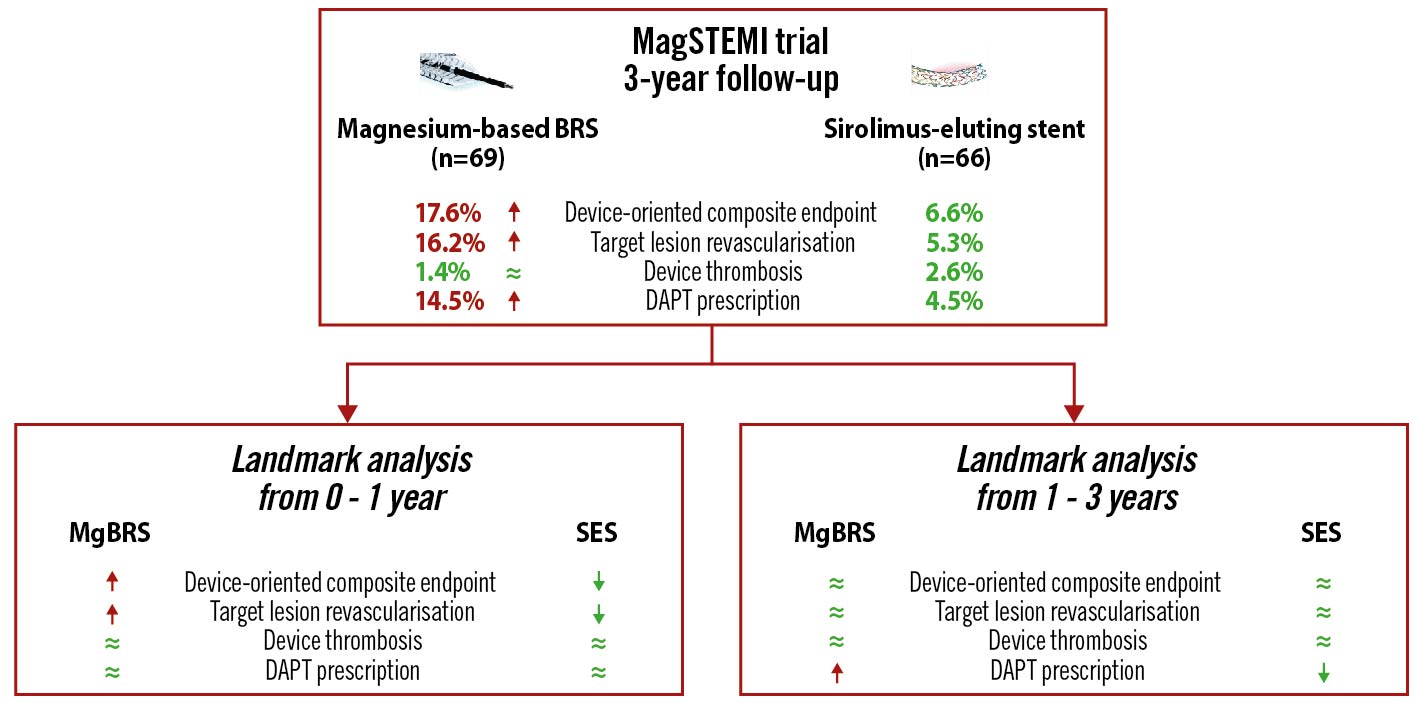
Central illustration. Three-year follow-up of the MAGnesium-based Bioresorbable Scaffold in ST-Segment Elevation Myocardial Infarction (MAGSTEMI; NCT03234348). The red arrow (⭡) denotes a statistically significant increase, the green approximation symbol (≈) denotes no statistical difference, and the green arrow (⭣) denotes a significant decrease. DAPT: dual antiplatelet therapy; MgBRS: magnesium-based bioresorbable scaffold; SES: permanent metallic sirolimus-eluting stent
Patients with STEMI have an increased risk of device-related adverse events mainly clustered within the 5 years of follow-up after primary PCI. However, beyond this period, non-device-related adverse events account for the majority of adverse events1. In the MAGSTEMI trial, we analysed the complete 3-year outcomes of patients treated with MgBRS versus SES. At 3-year follow-up, MgBRS were associated with a higher rate of DoCE compared to SES. This difference was mainly driven by an increased rate of TLR occurring within the first year. In the landmark analysis, there was a low rate of events in both groups. Between 1- and 3-year follow-up, there were no differences in the rate of DoCE between MgBRS and SES, neither in TLR nor in target vessel revascularisation.
Several OCT studies have suggested that the underlying mechanism of the MgBRS restenosis appeared to be the scaffold collapse31011. This highlights the need for increasing the radial force and resorption time of further iterations of this device, especially within the first year. Nevertheless, the underlying mechanism of late events is less well described with few reported cases4. In this regard, as complete resorption may be achieved at 1 year, with a possible average of 3 years, the absence of the scaffold following complete resorption and its biocompatibility may be an advantage of MgBRS1213.
Interestingly, MgBRS did not exhibit any safety concerns (i.e., scaffold thrombosis or target vessel myocardial infarction) in this thrombogenic setting, especially considering the low rate of DAPT. This finding is in line with data obtained from a preclinical swine model of an arteriovenous shunt, including permanent SES, MgBRS, and polymer-based BRS. In this model, MgBRS showed less platelet coverage (3.0%) than SES (4.6%) and polymer-based BRS (21.8%)12.
An earlier analysis of the MAGSTEMI trial reported the 1-year OCT findings of patients treated with MgBRS3. Scaffold discontinuities were observed in 10.4% of patients treated with MgBRS and 0% of those treated with SES. It should be noted that the effect of these 1-year scaffold discontinuity OCT findings on long-term clinical outcomes is uncertain. Accordingly, in this 3-year follow-up report, we found that the presence of scaffold discontinuities at 1 year was not associated with impaired 3-year clinical outcomes.
At 3-year follow-up, patients treated with MgBRS were more frequently on DAPT than those treated with SES, without differences in bleeding events. Moreover, of those patients in whom DAPT was prolonged, only 1 experienced a bleeding event between 1- and 3-year follow-up. The primary factor in the decision to prolong DAPT was the treating physician’s recommendation. In the polymeric BRS, the prolongation of DAPT up to the complete bioresorption (~3 years) was initially proposed to reduce the risk of device thrombosis1415. However, data from the ABSORB trials did not find any benefits of prolonging DAPT beyond 1 year5. According to the MgBRS bioresorption kinetics (~95% at 1 year) and the low incidence of device thrombosis events, prolonging DAPT beyond 1 year may not provide any additional benefits.
Ultimately, due to the high rate of early TLR events with the current generation of MgBRS and the low rate of device-related events with current generation SES, it is uncertain if the potential long-term benefit of the MgBRS will counterbalance its initial limitations. It is worth noting that this phenomenon was previously observed in the polymeric BRS16.
Limitations
A few limitations must be acknowledged. First, the trial was underpowered to sufficiently investigate secondary outcomes such as DoCE or PoCE components. Therefore, clinical outcomes can only be taken as hypothesis-generating. Secondly, DAPT was more often maintained at 3 years in the MgBRS group, mainly due to the decision of the treating physician. Therefore, we cannot rule out a protective role of DAPT on the low rate of thrombotic events at 3 years.
Conclusion
In conclusion, in the MAGSTEMI trial, MgBRS was associated with a higher rate of TLR, clustered within the first year of follow-up. A potentially favourable safety profile counterbalances this increased restenotic risk in terms of thrombotic events up to 3 years. Larger trials with new generation MgBRS powered to clinical events are warranted to understand this technology's potential.
Impact on daily practice
At long-term follow-up, the current generation of the magnesium-based bioresorbable scaffold (MgBRS) had a higher rate of device-oriented adverse outcomes compared to the permanent metallic sirolimus-eluting stent (SES) in STEMI patients. This difference was mainly driven by an increased rate of TLR occurring within the first year. It is worth noting that there were no differences between the MgBRS and the SES in the device thrombosis rate. With the current MgBRS and SES generations, it is uncertain if the potential long-term benefit of the MgBRS will counterbalance its initial limitations. An increase in the scaffolding duration and radial force of the device may contribute to overcoming the restenosis issues.
Funding
The funding source was the Spanish Heart Foundation. The promoter of the study had no role in the study design, providing only funds for independent data management (Effice SL, Madrid) and a core laboratory analysis centre (Barcicore, Cardiac Imaging Corelab, Barcelona) for database management and all statistical analyses. The authors had unrestricted access to the data and vouch for the accuracy and completeness of the data and analyses and for the fidelity of the study to the protocol. The corresponding author had final responsibility for the decision to submit for publication.
Conflict of interest statement
M. Sabaté is a consultant for Abbott Vascular and iVascular. A. Cequier receives grants and personal fees from Abbott Vascular, Medtronic, Boston Scientific, and Biosensors. P. Salinas receives speaker fees from Terumo, Boston Scientific, AlviMedica, and Biomenco. S. Brugaletta is a consultant for Boston Scientific and iVascular, and his institution received a research grant from AstraZeneca. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

