So far, bioresorbable scaffold (BRS) technology has failed in achieving non-inferiority compared to metallic drug-eluting stents (DES). While the polymeric Absorb (Abbott) bioresorbable vascular scaffold was rigorously tested against metallic DES and showed inferior efficacy and safety, any other BRS technology has undergone no or very limited assessment in powered randomised trials, including the previous two generations of resorbable magnesium scaffold (RMS). In the only randomised controlled trial (RCT), the small MAGSTEMI trial, the second-generation drug-eluting absorbable magnesium scaffold (DREAMS 2G, commercial name Magmaris [Biotronik]) showed a lower clinical and angiographic efficacy as compared to DES, yet a better vasomotor function at follow-up1. This trial and the results of the BIOSOLVE observational studies reportedly identified stent recoil due to limited radial strength as the leading reason for inferior efficacy. The latest iteration, the third-generation DREAMS (DREAMS 3G, commercial name Freesolve [Biotronik]), was developed to address this limitation. Compared to Magmaris, DREAMS 3G improved radial strength despite reduced strut thickness. In the first-in-human study (BIOMAG-I) assessing the DREAMS 3G, target lesion revascularisation at 12 months rarely occurred (2.6%) and no patient experienced target vessel myocardial infarction or stent thrombosis2. The angiographic late lumen loss (LLL) was 0.24±0.36 mm, which is numerically lower than that of Magmaris and comparable to Absorb, but higher as compared to Orsiro (Biotronik) (Figure 1A).
In this issue of EuroIntervention, Seguchi et al3 report on the serial intracoronary imaging evaluation of the DREAMS 3G that was assessed in the BIOMAG-I study2. The main BIOMAG-I report included clinical, angiographic and intravascular ultrasound (IVUS) data at 6 months and in a previous analysis published in this journal, IVUS and optical coherence tomography (OCT) data at 12 months were reported2. The specifics of this analysis include insights from serial postprocedural, 6-month and 12-month IVUS/OCT recordings. To start with the main limitation, it is notable that only 52% of patients were serially assessed with sufficient quality allowing for core lab analysis.
The key insight provided by OCT is the lack of stent remnants by OCT at 6 and 12 months, consistent with a preclinical study4. The absence of struts at follow-up suggests that late and very late scaffold thrombosis will rarely occur. Indeed, the Achilles heel of Absorb were non-homogeneously resorbing struts which were sometimes present for more than 3 years, occasionally protruding into the lumen and causing very late scaffold thrombosis as shown in the INVEST registry5.
Is the rapid dissolution of struts achieved at the price of significant lesion recoil? Looking at the LLL data, the DREAMS 3G improved as compared to previous-generation RMS. Yet, according to the serial IVUS analysis presented by Seguchi et al, the minimum lumen area after DREAMS 3G implantation decreased from 6.47 mm² to 4.95 mm² over one year in the BIOMAG-I study (Figure 1B), which is a greater decrease than was previously observed with the Absorb and Orsiro devices, although these devices were never compared with one other directly. Based on serial minimal stent area assessments by IVUS, the main contributor to lumen loss is recoil, although the magnitude is low.
The authors provide a detailed analysis of protruding neointimal tissue (PNT), i.e., neointimal tissue protruding into the lumen at the location of the stent struts. The struts are no longer visible at follow-up so that only protruding neointimal tissue remains. Although PNT is pronounced following DREAMS 3G, neointimal protrusion is also observed following DES, yet the stent of course remains inside the protruding neointima. The authors found more “favourable” imaging outcomes in lesions with high PNT, such as lower LLL and a smaller decrease in lumen area. The IVUS analysis, however, indicates that more PNT was found in large vessels (i.e., larger postprocedural lumen and vessel area) so that the more beneficial outcomes may simply be the function of baseline differences in vessel size. Larger vessels may lead to stents that are less embedded (or even more malapposed). Indeed, malapposition tended to be more frequent in the high PNT group. Larger vessels require larger stents, and larger DREAMS 3G devices have thicker struts, which reportedly lead to more neointimal proliferation. Interestingly, PNT decreases from 6 to 12 months, suggesting this to be a partially transient phenomenon. Together with the observation of a significant reduction in peristrut low intensity area, an OCT finding referring to inflammatory activity, the findings indicate favourable strut absorption with only transient inflammation and subsequent healing at 12 months.
What is the serial imaging data collectively suggesting? First, late and very late stent thrombosis will be a very unlikely event in the absence of struts. Second, the fast resorption process is not leading to excessive inflammation. Third, radial strength was successfully addressed with the latest device iteration, but lumen recoil may still occur and requires some attention by the operator. Lesion preparation likely continues to be relevant for DREAMS 3G implanters who should not only pay attention to calcified but also fibrotic lesions as they reportedly also cause elastic recoil6.
The decision to conduct the BIOMAG-II study (ClinicalTrials.gov: NCT05540223) to assess non-inferiority of the DREAMS 3G against the XIENCE DES (Abbott) in 1,859 patients is essential to assess whether BRS will be part of the future of revascularisation therapy. The serial imaging data suggests it may be so, but clinical evidence will have the last word.
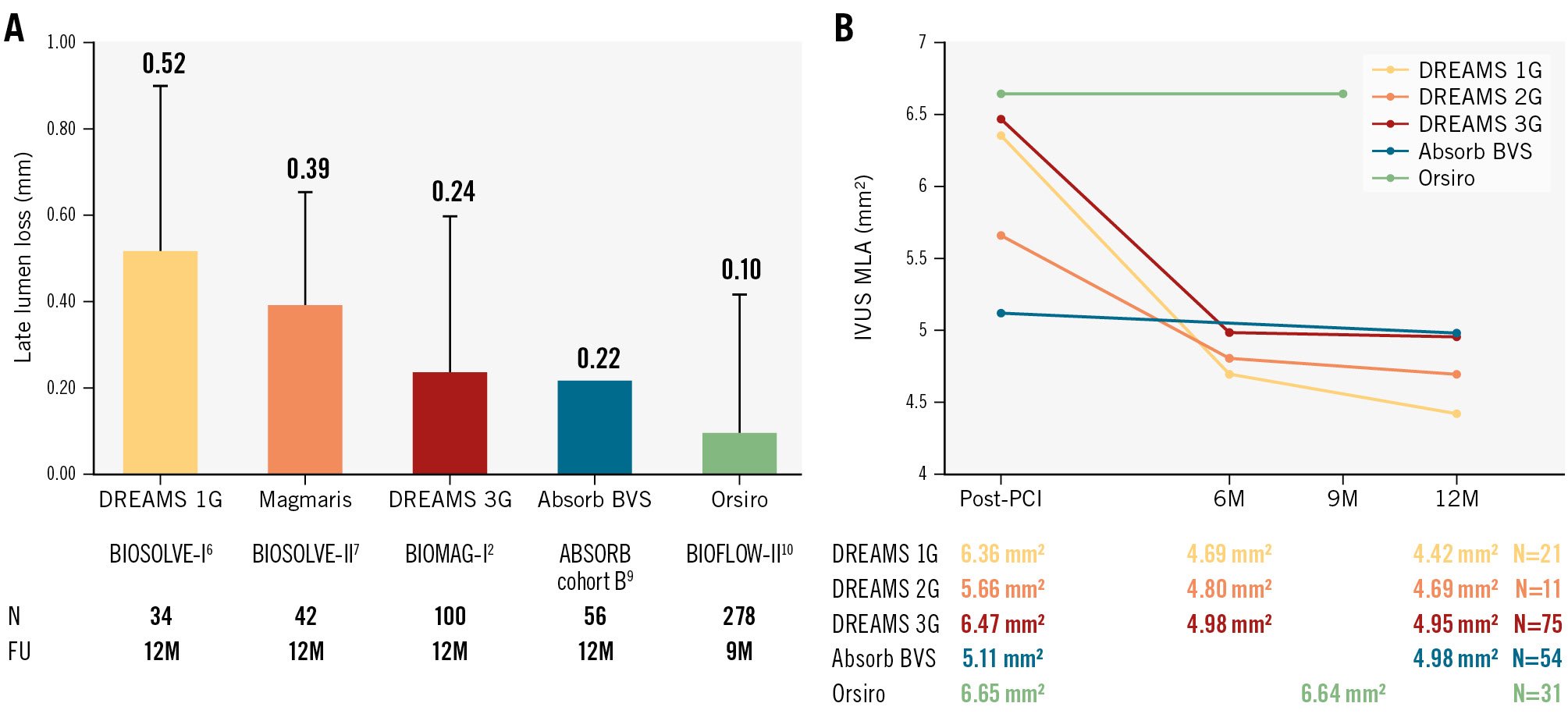
Figure 1. Angiographic and serial IVUS data from three generations of BRS, BVS and Orsiro are compared. Note that these data were derived from single-arm observational studies not directly comparing the devices against each other. FU: follow-up; IVUS: intravascular ultrasound; M: months; MLA: minimum lumen area; PCI: percutaneous coronary intervention
Conflict of interest statement
L. Räber has received research grants to the institution from Abbott, Biotronik, Boston Scientific, HeartFlow, Sanofi, and Regeneron; and has received speaker or consultation fees from Abbott, Amgen, AstraZeneca, Biotronik, Canon, Medtronic, Novo Nordisk, Occlutech, and Sanofi outside the submitted work. R. Kakizaki received consulting fees from Infraredx USA; speaker fees from Abbott Medical Japan, Boston Scientific Japan, Philips Japan, OrbusNeich Medical; and manuscript writing fees from OrbusNeich Medical and Philips Japan outside the submitted work.

