
Abstract
Aims: We aimed to assess the safety and performance of the DREAMS 2G scaffold up to 24 months post implant.
Methods and results: The present study population comprises a total of 184 patients with 189 lesions who were enrolled in the prospective, multicentre BIOSOLVE-II and BIOSOLVE-III trials. Clinical follow-up was scheduled at one, six, 12, 24 and 36 months. The present report includes pooled follow-up data at six months and BIOSOLVE-II data at 24 months. Patients were 65.5±10.8 years old, and lesions were 12.5±5.1 mm long with reference diameters of 2.7±0.4 mm. Procedural success was obtained in 97.8%. At six months, the composite clinical endpoint target lesion failure was 3.3% (95% CI: 1.2-7.1), based on two cardiac deaths (1.1%, one unknown and one not device-related), one target vessel myocardial infarction (0.6%), and three clinically driven target lesion revascularisations (1.7%). For BIOSOLVE-II at 24 months, the target lesion failure rate was 5.9% (95% CI: 2.4-11.8), based on two cardiac deaths (1.7%), one target vessel myocardial infarction (0.9%) and four target lesion revascularisations (3.4%). There was no definite or probable scaffold thrombosis.
Conclusions: The present analysis provides additional evidence on the safety of a drug-eluting absorbable metal scaffold with promising clinical outcomes up to 24 months and absence of definite or probable scaffold thrombosis.
Abbreviations
BRS: bioresorbable scaffold
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
DREAMS 2G: wdrug-eluting absorbable metal/magnesium scaffold second generation
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
PLLA: poly-L-lactic acid
TLF: target lesion failure
TLR: target lesion revascularisation
Introduction
Bioresorbable vascular scaffolds (BRS) have been developed to overcome limitations of bare metal and drug-eluting stents (DES), in particular to avoid creation of permanently caged vessel segments, chronic vessel wall inflammation or long-term stent crushing and fractures. Furthermore, BRS provide the option of non-invasive vessel lumen imaging by magnetic resonance or computed tomography and facilitate surgical or percutaneous repeat coronary revascularisation1,2.
Currently, three CE-marked drug-eluting BRS are available which are based on two different concepts of bioresorption: the polymeric Absorb (Abbott Vascular, Santa Clara, CA, USA) and DESolve® (Elixir Medical, Sunnyvale, CA, USA) scaffolds and the metal DREAMS 2G scaffold (Magmaris; Biotronik, Bülach, Switzerland).
The sirolimus-eluting absorbable metal scaffold DREAMS 2G is built on the experience gained from its predecessors, the bare absorbable metal scaffold (AMS) and the paclitaxel-eluting DREAMS 1G (Biotronik). Both devices were tested in the PROGRESS and BIOSOLVE-I studies. There was no target lesion failure (TLF) beyond 12-month follow-up and no scaffold thrombosis. In addition, in the few patients with angiographic follow-up beyond one year, angiographic parameters tended to improve. However, late lumen loss at early follow-up was unacceptable, showing that a prolonged scaffolding time was required3,4. Hence, DREAMS 2G was developed with a modified magnesium backbone, allowing a longer scaffolding time due to its slower degradation. Furthermore, the drug-polymer combination was modified to sirolimus/PLLA, the same combination that is used for the biodegradable Orsiro DES (Biotronik) with excellent clinical results5,6.
DREAMS 2G was first tested in the BIOSOLVE-II study with favourable 12-month outcomes7. However, as the true benefit of absorbable scaffolds is expected long-term, data beyond the resorption period are essential. This is even more necessary, as reports signalling very late thrombosis after implantation of the Absorb scaffold have been published recently8-10. We therefore assessed the safety and performance of DREAMS 2G in BIOSOLVE-II at 24 months. To provide additional data on a larger number of patients, we pooled the six-month results of BIOSOLVE-II with those of the BIOSOLVE-III study.
Methods
STUDY DESIGN AND POPULATION
The design of the BIOSOLVE-II study has been previously described7,11. BIOSOLVE-II and BIOSOLVE-III are both prospective, multicentre studies to evaluate the safety and performance of DREAMS 2G. BIOSOLVE-II is conducted in 13 institutions in Europe, South America and Asia, and BIOSOLVE-III in eight institutions in Europe.
Both studies comply with the Declaration of Helsinki, Good Clinical Practice, ISO14155, and were approved by the institutional ethics committees. All patients provided written informed consent.
The main patient inclusion criteria were: stable or unstable angina or documented silent ischaemia, a maximum of two single de novo lesions in two separate coronary arteries, reference vessel diameter of 2.2-3.7 mm for device diameters of 2.5 mm to 3.5 mm, lesion length ≤21 mm, and a diameter stenosis ≥50% and <100%. The main exclusion criteria were: thrombus in the target vessel, severe calcification, three-vessel disease, ostial target lesions within 5 mm of the vessel origin, target lesions involving a side branch >2.0 mm, target lesion located in or supplied by an arterial or venous bypass graft, and unsuccessful predilatation. The full lists of inclusion and exclusion criteria can be accessed at ClinicalTrials.gov (NCT01960504 and NCT02716220).
Clinical follow-up was planned at one, six and 12 months, and annually thereafter up to three years. Angiographic follow-up was scheduled at six months for BIOSOLVE-II and (to allow an angiographic analysis at the end of the absorption period) at 12 months for BIOSOLVE-III. BIOSOLVE-II included additional imaging with intravascular ultrasound and optical coherence tomography (OCT); these outcomes have been reported7,11.
STUDY DEVICE
DREAMS 2G has been described previously7,11,12. In brief, it consists of a balloon-expandable bioresorbable metal scaffold made from a magnesium alloy, premounted on a rapid exchange delivery system. The struts are 150 µm thick, have a width of 150 µm, and are laser-polished, and their surface is completely coated with bioresorbable PLLA that elutes sirolimus. The scaffold was available in diameters of 2.5, 3.0, and 3.5 mm and lengths of 20 and 25 mm for BIOSOLVE-II, and diameters of 3.0 and 3.5 mm, and lengths of 15, 20 and 25 mm for BIOSOLVE-III.
ENDPOINTS
The primary endpoints were late lumen loss at six-month follow-up (BIOSOLVE-II) and procedure success (BIOSOLVE-III). Procedure success was defined as final diameter stenosis of <30% by quantitative coronary angiography without occurrence of in-hospital death, Q-wave or non-Q-wave myocardial infarction or repeat revascularisation of the target lesion. Secondary endpoints were target lesion failure (TLF), a composite of cardiac death, target vessel myocardial infarction13, or clinically driven target lesion revascularisation (TLR), and scaffold thrombosis14. Secondary angiographic endpoints were reported previously7,11.
A clinical events committee adjudicated all adverse events, and angiographic data were obtained from analyses of an independent core laboratory.
PROCEDURE
DREAMS 2G was implanted after a mandatory predilatation. The size of the predilatation balloon had to be ≤0.5 mm smaller than the reference vessel diameter but not larger than the reference vessel; its length had to be shorter than or the same as the lesion length.
Only one study device per lesion was allowed, although in bail-out situations a second DREAMS 2G could be used and, in case of failure, an Orsiro DES. Post-dilatation could be performed at the discretion of the investigator, but the maximum inner diameter of the DREAMS 2G (as indicated on the label) was not permitted to be exceeded. In addition, the post-dilatation balloon had to be shorter than the scaffold. Dual antiplatelet therapy was recommended for a minimum of six months.
STATISTICAL ANALYSIS
Data are presented using descriptive statistical methods. For continuous variables, means±standard deviations are presented. For categorical data, absolute and relative frequencies are reported. When appropriate, 95% confidence intervals were calculated. All statistical analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Between October 2013 and May 2015, 123 patients were enrolled in the BIOSOLVE-II study and, between March and September 2016, 61 patients in BIOSOLVE-III. In two BIOSOLVE-II patients, DREAMS 2G could not be implanted due to insufficient predilatation; these patients were only counted for procedure success but excluded from follow-up. Treatment of two lesions was performed in five patients, resulting in 189 lesions overall. Follow-up data were available for 180/182 BIOSOLVE-II and BIOSOLVE-III patients at six months (98.9%) and for 120/121 BIOSOLVE-II patients at 24 months (99.2%) (Figure 1).
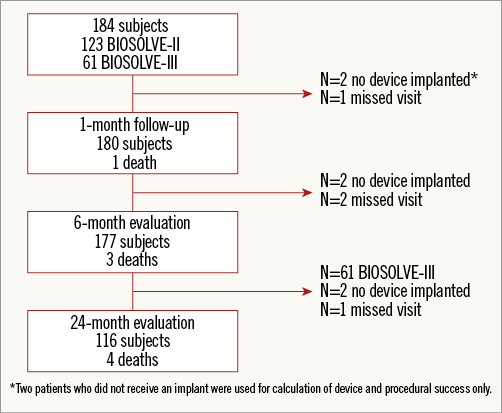
Figure 1. Study flow chart. Two patients of BIOSOLVE-II did not receive an implant and were counted for procedural success only.
Baseline characteristics are listed in Table 1. Patients were 65.5±10.8 years old, 63.6% (117/184) were male, 79.3% (146/184) had hypertension and 25.0% (46/184) were diabetics.
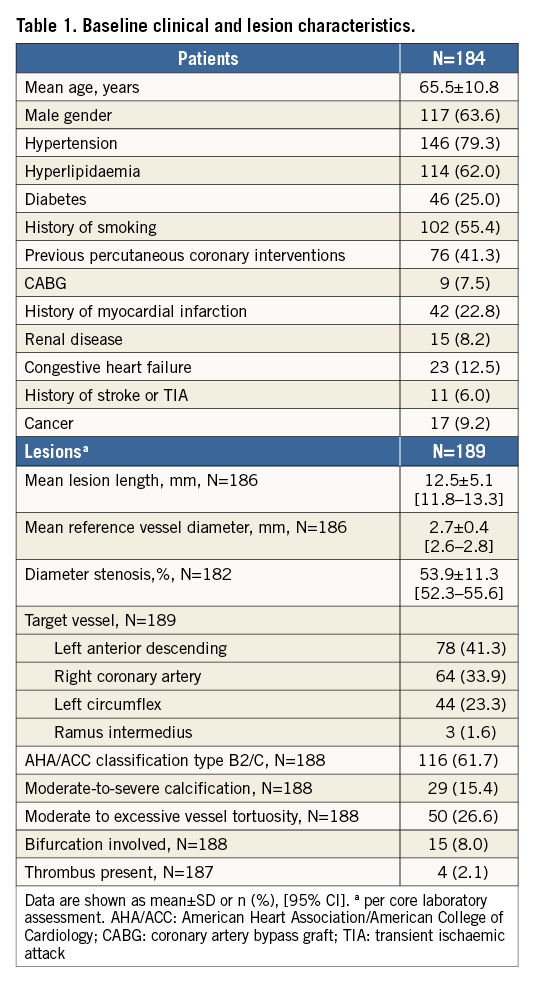
Vessels were 12.5±5.1 mm long with a mean reference diameter of 2.7±0.4 mm. Mandatory predilatation was performed in all lesions. One to five inflations per balloon were performed (mean 1.5±0.9), and in 28 lesions (14.8%) more than one predilatation balloon was needed. Predilatation balloons were 2.9±0.4 mm in diameter, ranging from 2 to 4 mm, and the maximal pressure applied was 14.7±4.2 atm, ranging from 4 to 26 atm. More than one scaffold was implanted in seven patients (six for dissections, one as the original scaffold was too short to cover the lesion), and post-dilatation of scaffolds was performed in 69.0%. Procedure success was achieved in 97.8% (180/184).
At baseline, stable angina was present in 75.0% (138/184) and unstable and documented silent ischaemia in 12.5% (23/184) each. At one month, 94.4% (170/180) of patients were symptom-free; the remaining patients had stable angina. At six months, 88.2% (149/169) of patients were symptom-free while 9.5% had stable angina, 1.8% unstable angina and 0.6% documented silent ischaemia. Dual antiplatelet therapy was stopped in 2.7% prior to six months and in 86% prior to 24 months.
Target lesion failure at six months occurred in six patients (3.3% [95% CI: 1.2-7.1]), consisting of two cardiac deaths (1.1%, 95% CI: 0.1-3.9]), one target vessel myocardial infarction (0.6% [95% CI: 0.0-3.0]), and three clinically driven TLR (1.7% [95% CI: 0.3-4.8%]) (Table 2). Figure 2 presents the case with TLR at day 84. Data beyond six months are available for the BIOSOLVE-II population only. Between six and 24 months, one additional cardiac death and two additional clinically driven TLR occurred, resulting in a TLF rate of 5.9% (n=7 [95% CI: 2.4-11.8]). Details of TLF events are provided in Table 3. No definite or probable scaffold thrombosis was observed. Figure 3 presents the case of a patient who already had serial imaging follow-up up to three years.
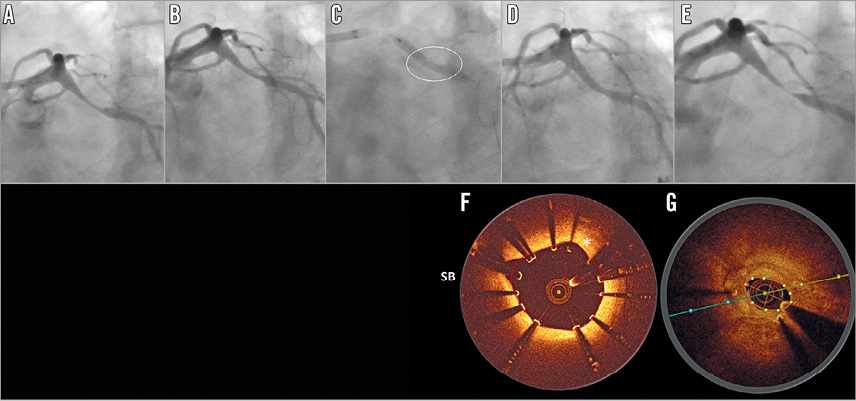
Figure 2. Angiographic and OCT results of a case that required target lesion revascularisation. Angiographic images of the left circumflex (A) pre-procedure, (B) after predilatation, (C) after scaffold implantation (the circle marks the area of incomplete expansion), (D) post procedure, and (E) at 84 days post implant. (F) Post-procedure OCT image (incomplete strut apposition from 9 to 11 o’clock, 330 microns), and (G) at 84 days post implant (marked compression in the area of the original eccentric lesion with significant neointimal hyperplasia). OCT: optical coherence tomography; SB: side branch
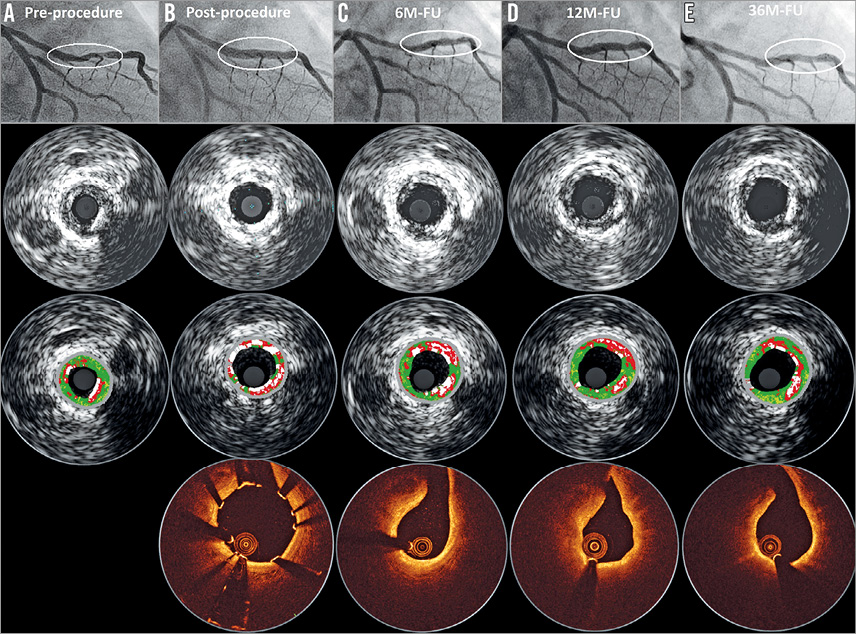
Figure 3. Serial quantitative angiographic, intravascular ultrasound and OCT results of a patient with DREAMS 2G implantation. A) A lesion in the mid left anterior descending (type B2, length 14 mm, diameter 2.8 mm, diameter stenosis 80% by visual estimate). Predilatation was conducted with a 3.0×12 mm semi-compliant balloon. Thereafter, a DREAMS 2G scaffold (3.0×20 mm) was implanted. Post-dilatation was performed with a 3.0×12 mm semi-compliant balloon. B) Post-procedure OCT and intravascular ultrasound demonstrate a good conformability to the vessel and a metallic appearance of DREAMS 2G. C) At six months, the degradation of the scaffold is detectable, and the struts covering the side branch have disappeared. D) At 12 months, OCT shows homogeneous neointima formation. E) Preservation of the lumen without any restenosis at 36 months. FU: follow-up; M: month; OCT: optical coherence tomography
Discussion
This is the first report of DREAMS 2G with data up to two years. These data are particularly important as approximately 95% of the magnesium scaffold is expected to be absorbed within 12 months11. The main findings of our series are low TLF rates at six and 24 months comparable to conventional DES, and the absence of definite or probable scaffold thrombosis.
The incidence of TLF, a composite of cardiac death, target vessel myocardial infarction or clinically driven TLR, was 3.3% at six months, which is similar to the rate of 3.3% for the DESolve BRS15. In a recent meta-analysis of more than 8,000 Absorb BRS with a mean follow-up time of six months, cardiac death occurred in 0.6%, myocardial infarction in 2.1% and TLR in 2.0% of patients16, compared to 1.1%, 0.6% and 1.7% in DREAMS 2G in our present series.
At 24 months, we found a TLF rate of 5.9% in DREAMS 2G, which compares favourably with the TLF rate for DESolve (7.4%) in the DESolve Nx trial15. In the ABSORB Japan, ABSORB II, ABSORB III, and AIDA studies, 24-month TLF rates for the Absorb BRS were 7.3%, 7.0%, 11.0%, and 10.3%, respectively. In comparison, the TLF rates for the comparator, an everolimus-eluting cobalt-chromium stent, were 3.8%, 3.0%, 7.9%, and 8.9% in the four aforementioned trials, respectively10,17-19.
The ESC-EAPCI report on the evaluation of coronary stents reported the following average event rates for new-generation DES at nine to 12 months20: cardiac death 1.0%, myocardial infarction 2.89%, TLR 2.91% and definite stent thrombosis 0.47%. The six- and even 24-month data of DREAMS 2G are comparable. In particular, the cardiac death rate was 1.1% at six months for the pooled analysis and 1.7% at 24 months for BIOSOLVE-II, while the corresponding rates of myocardial infarction were 0.6% and 0.9%, and of TLR 1.7% and 3.4%, respectively.
While, in accordance with the results in precursor metallic scaffolds3, no myocardial infarction beyond the 12-month follow-up was observed, there were two TLR beyond 12 months. The two TLR occurred on days 461 and 561 post procedure with angiographic diameter stenoses of 50% and 67% (per core laboratory); both patients were on DAPT, and the corresponding diameter stenoses at six months had been 43% and 32%.
The fact that no definite or probable scaffold thrombosis was observed is encouraging, and is in line with the absence of scaffold thrombosis for previous versions of metal absorbable scaffolds3,4. Notably, DAPT was recommended for at least six months post procedure in our series while for some more recent trials with polymeric scaffolds ≥12 months of DAPT was recommended10,21. Potential reasons for the absence of scaffold thrombosis have been discussed previously7,21. In brief, no intraluminal mass was detected by optical coherence tomography in BIOSOLVE-II at six and 12 months and no malapposed struts were detected at six months, when scaffold struts were already well embedded into the vessel wall (Figure 3). With its 95% absorption at 12 months, a late acquired malapposition of our present metal scaffold is unlikely. Furthermore, the surface of the laser-polished DREAMS 2G is smooth, and strut cross-sections are rectangular with rounded edges, which may facilitate embedding into the vessel wall.
A recent study in porcine and rabbit models showed an increased endothelialisation and decreased thrombus formation for DREAMS 2G compared to Absorb. Inflammation for DREAMS 2G peaked at 90 days and decreased thereafter; at one and two years, inflammation was lower for DREAMS 2G versus an everolimus-eluting cobalt-chromium stent22. Furthermore, recent in vitro tests showed an improved deliverability of DREAMS 2G as compared to the Absorb BRS due to the metallic properties of DREAMS 2G, with less bending stiffness despite higher radial strength, indicating a better vessel conformability and no time-dependent recoil of DREAMS 2G in contrast to Absorb and DESolve23.
Although the recently propagated “4P” strategy (patient selection, predilatation, proper sizing and post-dilatation) was not implemented in both studies, the outcomes of BIOSOLVE-II and BIOSOLVE-III are favourable. In Figure 2, the case example of a restenosis shows that the development of the restenosis may be related to insufficient predilatation (panel B); during scaffold implantation, the balloon was not fully expanded at the site of the previous eccentric stenosis (panel C). Furthermore, the post-procedure angiographic image (panel D) suggests undersizing of the scaffold and insufficient post-dilation, which is also supported by the respective OCT frame that showed malapposed and non-embedded struts (panel F). Finally, during treatment of the restenosis, a 3.5 mm DES failed to gain full expansion, requiring post-dilatations with non-compliant balloons up to a nominal diameter of 4.5 mm.
Meanwhile, predilatation with a non-compliant balloon in a 1:1 balloon-to-artery ratio is recommended. Moreover, post-dilatation with a non-compliant balloon up to 0.5 mm larger than the implanted scaffold with pressures >16 atm is recommended unless an optimal implantation result is confirmed by intracoronary imaging. The current treatment recommendations are summarised in a recently published preliminary expert consensus paper for metal scaffolds that particularly emphasises meticulous vessel preparation, precise sizing, imaging-guided implantation during the initial learning curve, and careful deployment and assessment of the scaffold12.
Limitations
Our series has several limitations. The fact that predominantly patients with limited clinical and anatomical complexity were included restricts the study interpretation to these patient and lesion characteristics. The lack of a control arm hampers the comparison to other devices; a randomised controlled trial would be needed. Furthermore, data on more patients are required to allow a robust assessment of rare events such as scaffold thrombosis. Routine angiographic follow-up at 24 months would have been of great interest. At least, we amended the current BIOSOLVE-II study protocol to obtain angiographic follow-up data at three years.
Conclusions
The present analysis provides additional evidence on the safety of DREAMS 2G (Magmaris; Biotronik) in a population with predominantly non-complex lesions. Clinical outcomes are favourable with low TLF rates up to two years. The absence of definite or probable scaffold thrombosis is promising, but requires confirmation in larger patient cohorts.
| Impact on daily practice The DREAMS 2G metal BRS has obtained CE approval in June 2016 with the commercial name Magmaris. Our data add further confirmation of the safety and performance of this novel scaffold: in particular (with the limitation of only 182 patients implanted), no definite or probable scaffold thrombosis was observed. Utmost care should be taken with respect to patient selection, device sizing, sufficient lesion preparation and post-dilatation to ensure good clinical outcomes with this novel technology. |
Acknowledgements
We thank Beatrix Doerr for her expert medical writing assistance.
Funding
This study was funded by Biotronik AG, Bülach, Switzerland.
Conflict of interest statement
M. Haude reports study grants and lecture fees from Biotronik, Abbott Vascular, Cardiac Dimensions, Medtronic, Volcano, and Lilly. C. von Birgelen reports institutional research grants from AstraZeneca, Biotronik, Boston Scientific, and Medtronic, and that he has been an unpaid consultant to device manufacturing companies, among them Biotronik. A. Abizaid is a consultant to and has received research grants from Boston Scientific, Abbott Vascular, Elixir Medical and Reva Medical. N.M. Van Mieghem and the Erasmus Medical Center have received research grants from Abbott Vascular, Boston Scientific, Medtronic, Claret Medical and PulseCath. S. Verheye is a consultant of Elixir and Neovasc. R. Tölg reports receiving consultant fees and speakers honoraria from Abbott Vascular and Biotronik. R. Waksman is a consultant of Abbott Vascular, Amgen, Biosensors International, Biotronik, Boston Scientific, Corindus, Lifetech Medical, Medtronic Vascular, Philips Volcano, and Symetis, acts for the speakers bureau of AstraZeneca, and receives grant support from Biosensors International, Biotronik, Boston Scientific, Edwards Lifesciences, and Abbott Vascular. The other authors have no conflicts of interest to declare.

