Abstract
Background: Bioresorbable scaffolds have been developed to overcome the limitations of drug-eluting stents and to reduce long-term adverse events.
Aims: We aimed to assess the long-term safety and efficacy of a sirolimus-eluting resorbable magnesium scaffold to ensure its safe rollout into clinical routine.
Methods: BIOSOLVE-IV is a prospective, international, multicentre registry including more than 100 centres in Europe, Asia, and Asia-Pacific. Enrolment started directly after the commercialisation of the device. Follow-up assessments are scheduled at 6 and 12 months, and annually for up to 5 years; we herein report the 24-month outcomes.
Results: Overall, 2,066 patients with 2,154 lesions were enrolled. Patients were 61.9±10.5 years old, 21.6% had diabetes, and 18.5% had non-ST-elevation myocardial infarction (NSTEMI). Lesions were 14.8±4.0 mm long with a reference vessel diameter of 3.2±0.3 mm. Device and procedure success were 97.5%, and 99.1%, respectively. The 24-month target lesion failure (TLF) rate was 6.8%, mainly consisting of clinically driven target lesion revascularisations (6.0%). Patients with NSTEMI had significantly higher TLF rates than those without (9.3% vs 6.2%; p=0.025), whereas there were no significant differences observed for patients with diabetes or with type B2/C lesions (a 24-month TLF rate of 7.0% and 7.9%, respectively). The 24-month rate of definite or probable scaffold thrombosis was 0.8%. Half of the scaffold thromboses occurred after premature discontinuation of antiplatelet/anticoagulation therapy, and only one scaffold thrombosis occurred beyond the 6-month follow-up, on day 391.
Conclusions: The BIOSOLVE-IV registry showed good safety and efficacy outcomes, confirming a safe rollout of the Magmaris into clinical practice.
Introduction
Second-generation drug-eluting stents (DES) have reduced restenosis rates compared to bare metal stents but have been associated with an increased risk of late events, especially late thrombotic events. These complications could be limited by prolonged dual antiplatelet therapy (DAPT) and refinements in stent designs but are still associated with a steady annual complication rate without a decline12.
Bioresorbable scaffolds (BRS) have been developed to overcome the limitations of permanent stents, to avoid the creation of permanently caged vessel segments with inhibition of vasomotion and vessel remodelling, and to avoid chronic vessel wall inflammation or long-term stent crushing and fractures1. The Absorb (Abbott Vascular) polymeric bioresorbable scaffold was the first European conformity (CE)-marked BRS. Initial short-term data were encouraging; however, the optimism associated with these preliminary studies was tempered by the results of the ABSORB II and III Trials. Finally, as a result of safety concerns (mainly increased rates of scaffold thrombosis), this technology was withdrawn from commercial use2.
In contrast to polymeric scaffolds, magnesium-based scaffolds have the advantage of a metal stent-like behaviour with good radial strength, low acute recoil, and laser polishing for a smooth surface that facilitates embedding into the vessel wall, reducing wall shear stress and thrombogenicity13.
The second-generation magnesium-based sirolimus-eluting scaffold Magmaris (BIOTRONIK) has shown promising outcomes in the controlled study environment of the BIOSOLVE-II and -III Studies1. To enable the safe rollout of this device into clinical practice, the BIOSOLVE-IV registry was initiated immediately after the obtention of the CE mark to collect long-term data on a large patient population, with endpoints powered for 12-month target lesion failure (TLF) and definite or probable scaffold thrombosis, and to comply with the call for more data from the European Society of Cardiology (ESC) and the European Association of Percutaneous Cardiovascular Interventions2. The 12-month outcomes of the first cohort of 1,075 patients, powered for the outcome of TLF, have been published previously3. To allow for an endpoint powered for device thrombosis, a second cohort was added, bringing the total to 2,066 patients. We herein report the 24-month outcomes of the full cohort. In addition, we assess the outcomes for the high-risk subgroups with type B2/C lesions, diabetes, and non-ST-elevation myocardial infarction (NSTEMI)456.
Methods
Study design and patients
The BIOSOLVE-IV registry has been described previously3. In brief, it is an international, multicentre, prospective registry with clinical follow-up at 6 and 12 months, and annually thereafter for 5 years. It is being conducted at 106 sites in Europe, Asia and Asia-Pacific.
Adult patients with a maximum of two single de novo lesions in two different major epicardial vessels ≤21 mm in length and between 2.7 and 3.7 mm in diameter were eligible. Patients with ST-elevation myocardial infarction, restenotic target lesion, and left main disease were excluded. The full list of in- and exclusion criteria is available at ClinicalTrials.gov: NCT02817802.
The registry was conducted according to the current version of the Declaration of Helsinki, ISO14155, where applicable, and local guidelines; it was approved by the institutional ethics committees. All patients provided informed consent prior to any study procedure. The registry was monitored, and a clinical events committee member adjudicated all events for which a device relationship could not be ruled out.
Device and procedure
The Magmaris is a scaffold system consisting of a balloon-expandable bioresorbable scaffold premounted on the balloon of a rapid exchange percutaneous transluminal coronary angioplasty catheter. The scaffold backbone is made from bioresorbable magnesium, a material with high biocompatibility. Magnesium ions have several positive effects, such as decreased thrombogenicity, vasodilatation, inhibition of arterial wall stiffening processes and attenuation of vascular calcifications7.
The surface of the scaffold backbone is completely coated with bioresorbable poly-L-lactic acid (PLLA), which incorporates sirolimus and which is identical to the coating of the Orsiro (BIOTRONIK) DES3.
Implantation recommendations have been described previously3 and followed the “4P” strategy8; the use of intravascular ultrasound (IVUS) guidance was optional and left to the discretion of the implanting physician. DAPT had to be administered for at least 6 months.
Endpoints
The primary endpoint was TLF at 12 months, a composite of cardiac death, target vessel Q-wave or non-Q-wave myocardial infarction (MI), emergent coronary artery bypass graft (CABG), and clinically driven target lesion revascularisation (TLR). Procedural MI were adjudicated according to the Society for Cardiovascular Angiography and Interventions (SCAI) definition9, and spontaneous MI according to the extended historical definitions10. The secondary endpoints were TLF at timepoints other than 12 months and its individual components: clinically driven target vessel revascularisation (TVR), scaffold thrombosis11, procedure success and device success.
Statistical analysis
The sample size calculation has been reported previously3. A minimum of 1,065 patients were included in the calculation of the first cohort (endpoints of 12-month TLF, null hypothesis ≥10%, weighted mean 6.6%, and 95% power), and 2,054 patients for the full cohort (endpoints of 12-month definite or probable scaffold thrombosis, null hypothesis ≥1.49%, weighted mean 0.65%, and 90% power), both with a normal approximation to the binominal test, significance level α=0.025 (one-sided) and an expected dropout rate of 20%.
The analysis was performed for the intention-to-treat population and is based on data available. For quantitative variables, the mean values and standard deviation were calculated, and for qualitative variables, absolute and relative frequencies. For individual and combined clinical outcomes, the Kaplan-Meier estimator and 95% confidence intervals (CI) were calculated. In a post hoc analysis, outcomes in specific high-risk groups were compared using the Chi-square test, the t-test, Fisher’s exact test, and the log-rank test. No alpha-adjustment for multiple testing was performed for post hoc subgroup analyses, which are considered exploratory and hypothesis-generating. For exploratory analyses, a multivariable Cox regression model was performed for variables that showed an effect of p<0.15 in univariable Cox regression, to evaluate the adjusted hazard ratios. All analyses were conducted using SAS version 9.4 (SAS Institute).
Results
From September 2016 until July 2020, 2,066 subjects with 2,154 lesions were enrolled. Patients were 61.9±10.5 years on average, ranging from 26 to 86 years, 21.6% (n=446) had diabetes, 18.5% (n=383) had NSTEMI, and 0.4% (n=8) had ST-elevation myocardial infarction. The mean lesion length was 14.8±4.0 mm, with a mean reference vessel diameter of 3.2±0.3 mm (Table 1, for subgroups Supplementary Table 1).
Pre- and post-dilatation were performed in nearly all lesions (99.8% and 95.7%, respectively) (Table 2, for subgroups Supplementary Table 2). Nine subjects, in whom the Magmaris system entered the guiding catheter but could not be implanted at the target lesion, were only considered for device and procedural success analyses and were exempt from follow-up. When multiple scaffolds were implanted (n=65), they were implanted end-to-end in 61.5% of cases (n=40), overlapping in 32.3% (n=21), and “other” in 6.2% (n=4).
At follow-up, approximately 90% of patients were symptom-free, around 10% had stable angina, and unstable angina or documented silent ischaemia was present in less than 1% (Figure 1). In terms of antiplatelet therapy, at 12 months, 72.7% of patients were on DAPT therapy and 18.7% were on acetylsalicylic acid, whilst at 24 months, 21.3% were on DAPT and 54.6% on acetylsalicylic acid.
Follow-up data were available for 96.8% of patients at 24 months (Figure 2). The 24-month Kaplan-Meier estimate for TLF is 6.8%, predominantly consisting of clinically driven TLR (6.0%) (Table 3). The subgroup of NSTEMI patients had a significantly higher TLF rate compared with non-NSTEMI patients (9.3% vs 6.2%; p=0.025), based on a significantly higher rate of target vessel MI (3.7% vs 1.1%; p=0.0002) and a numerically higher rate of clinically driven TLR (8.0% vs 5.6%; p=0.063). Furthermore, clinically driven TVR occurred more often in the NSTEMI group (9.9% vs 6.1%; p=0.007). Likewise, multivariate analysis revealed NSTEMI as the only predictor for TLF (Supplementary Table 3).
There was no significant difference detected for TLF in diabetic versus non-diabetic patients (7.0% vs 6.7%; p=0.770) or for type B2/C lesions compared to type A/B1 lesions (7.9% vs 6.6%; p=0.360) (Central illustration, Table 3).
Definite or probable scaffold thrombosis occurred in 17 patients (0.8%, 0.4% excluding cases with early antiplatelet or anticoagulation interruption) and thus, the null hypothesis was rejected. Out of the 17 scaffold thromboses, only one occurred beyond the 6-month follow-up time window, on day 391 (four scaffold thromboses were subacute, one was very late [day 391] and the remaining were late).
Table 1. Baseline characteristics.
| N=2,066 patients | ||
|---|---|---|
| Age, years | 61.9±10.5 [61.4-62.3] | |
| Male | 1,539 (74.5) | |
| Hypertension | 1,370 (66.3) | |
| Hyperlipidaemia | 1,347 (65.2) | |
| Diabetes | 446 (21.6) | |
| Insulin-dependent | 94 (21.1) | |
| Renal disease | 126 (6.1) | |
| History of smoking | 1,224 (59.2) | |
| Current smoker | 513 (41.9) | |
| Previous PCI | 594 (28.8) | |
| Coronary artery bypass graft | 13 (0.6) | |
| History of myocardial infarction | 448 (21.7) | |
| NSTEMI | 383 (18.5) | |
| STEMI | 8 (0.4)* | |
| Ischaemic status | Stable angina | 998 (48.3) |
| Unstable angina | 358 (17.3) | |
| Silent ischaemia | 318 (15.4) | |
| N=2,154 lesions | ||
| Lesion length, mm | 14.8±4.0 [14.6-15.0] | |
| Reference vessel diameter, mm | 3.2±0.3 [3.2-3.2] | |
| Diameter stenosis, % | 82.2±10.6 [81.8-82.7] | |
| Target vessel | Left anterior descending | 1,067 (49.5) |
| Left circumflex artery | 442 (20.5) | |
| Right coronary artery | 620 (28.8) | |
| Ramus intermedius | 25 (1.2) | |
| AHA/ACC classification | Type A | 813 (37.7) |
| Type B1 | 1,012 (47.0) | |
| Type B2 | 255 (11.8) | |
| Type C | 74 (3.4) | |
| Calcification | Moderate to severe | 162 (7.5) |
| Tortuosity | Little tortuosity | 1,886 (87.6) |
| Moderate tortuosity | 255 (11.8) | |
| Excessive tortuosity | 13 (0.6) | |
| Bifurcation involved | 99 (4.6) | |
| Thrombus present | 3 (0.1) | |
| Data are mean±SD [95% CI], or n (%). *Violation of exclusion criteria. AHA/ACC: American Heart Association/American College of Cardiology; CI: confidence interval; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-elevation myocardial infarction | ||
Table 2. Procedural characteristics.
| N=2,154 lesions | |
|---|---|
| Predilatation performed | 2,150 (99.8) |
| Maximum pressure applied, atm, N=2,874 | 14.6±3.4 [14.5-14.7] |
| Scaffold length, mm, N=2,207 | 19.5±3.9 [19.3-19.6] |
| Scaffold diameter, mm, N=2,207 | 3.3±0.3 [3.2-3.3] |
| Maximum pressure applied, atm, N=2,195 | 14.4±2.7 [14.2-14.5] |
| Post-dilatation performed | 2,061 (95.7) |
| Maximum pressure applied, atm, N=2,295 | 17.1±3.4 [16.9-17.2] |
| Device success, N=2,231 stents | 2,177 (97.5) |
| Procedure success, N=2,062 patients | 2,047 (99.1) |
| Data are shown as mean±SD [95% CI] or n (%). Device success was defined as a final diameter stenosis of <30% using the assigned device only, successful delivery of the scaffold, appropriate scaffold deployment, and successful removal of the delivery system. Procedure success was defined final diameter stenosis <30% without the occurrence of death, myocardial infarction, or repeat target lesion revascularisation during the hospital stay. CI: confidence interval; SD: standard deviation | |
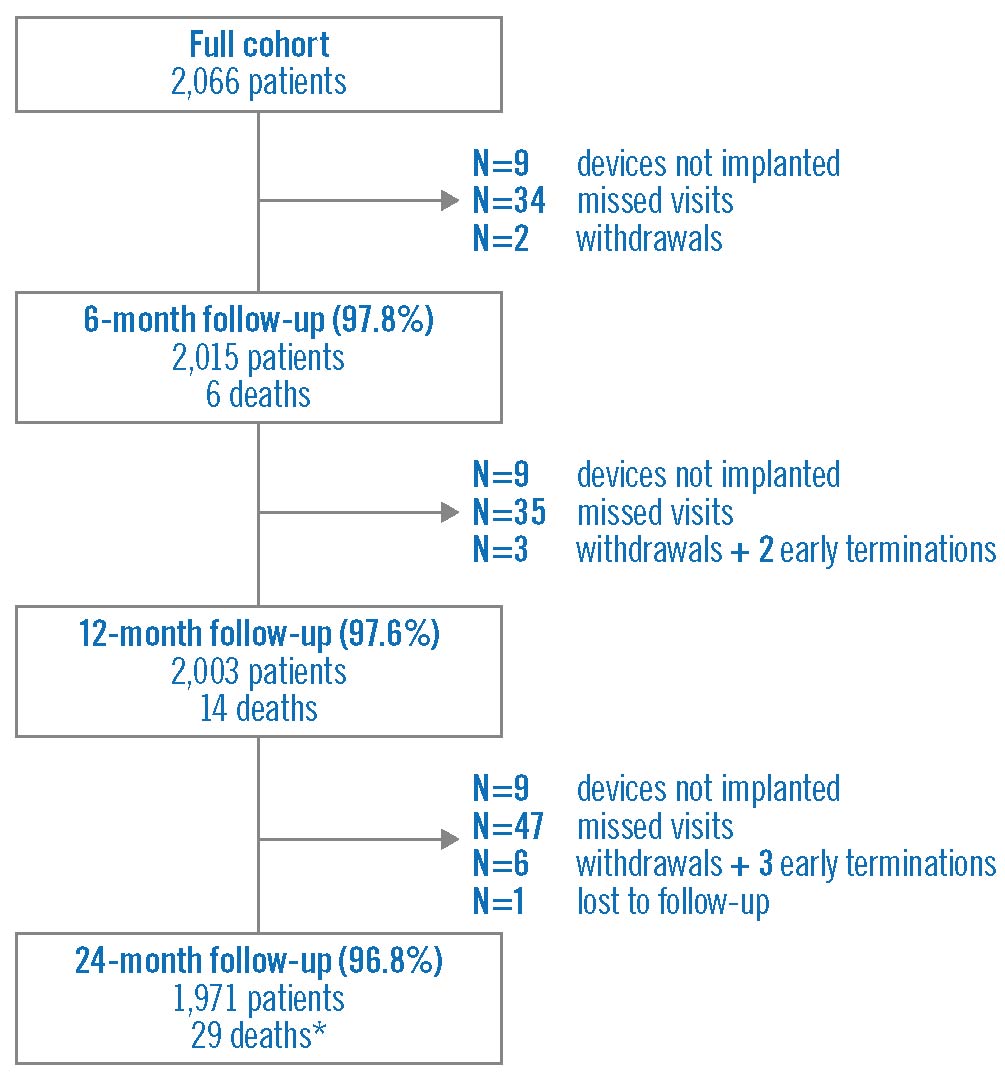
Figure 1. Ischaemic status at baseline and follow-up. Data were available for 2,066 patients at baseline, 2,015 patients at 6 months, 2,003 patients at 12 months, and 1,967 patients at 24 months.
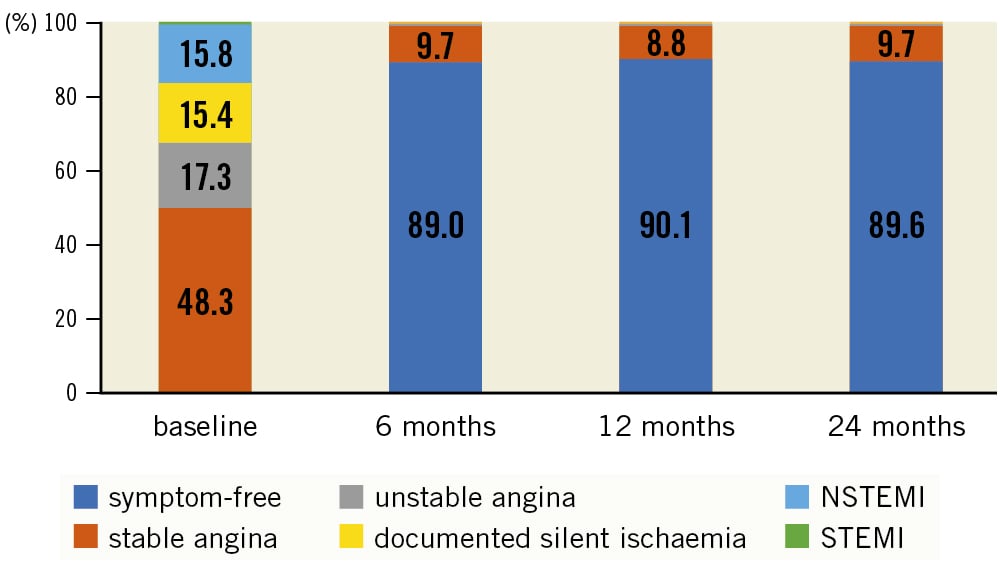
Figure 2. Patient flowchart. *One patient attended the 24-month visit, but died thereafter (resulting in 30 deaths overall). NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction
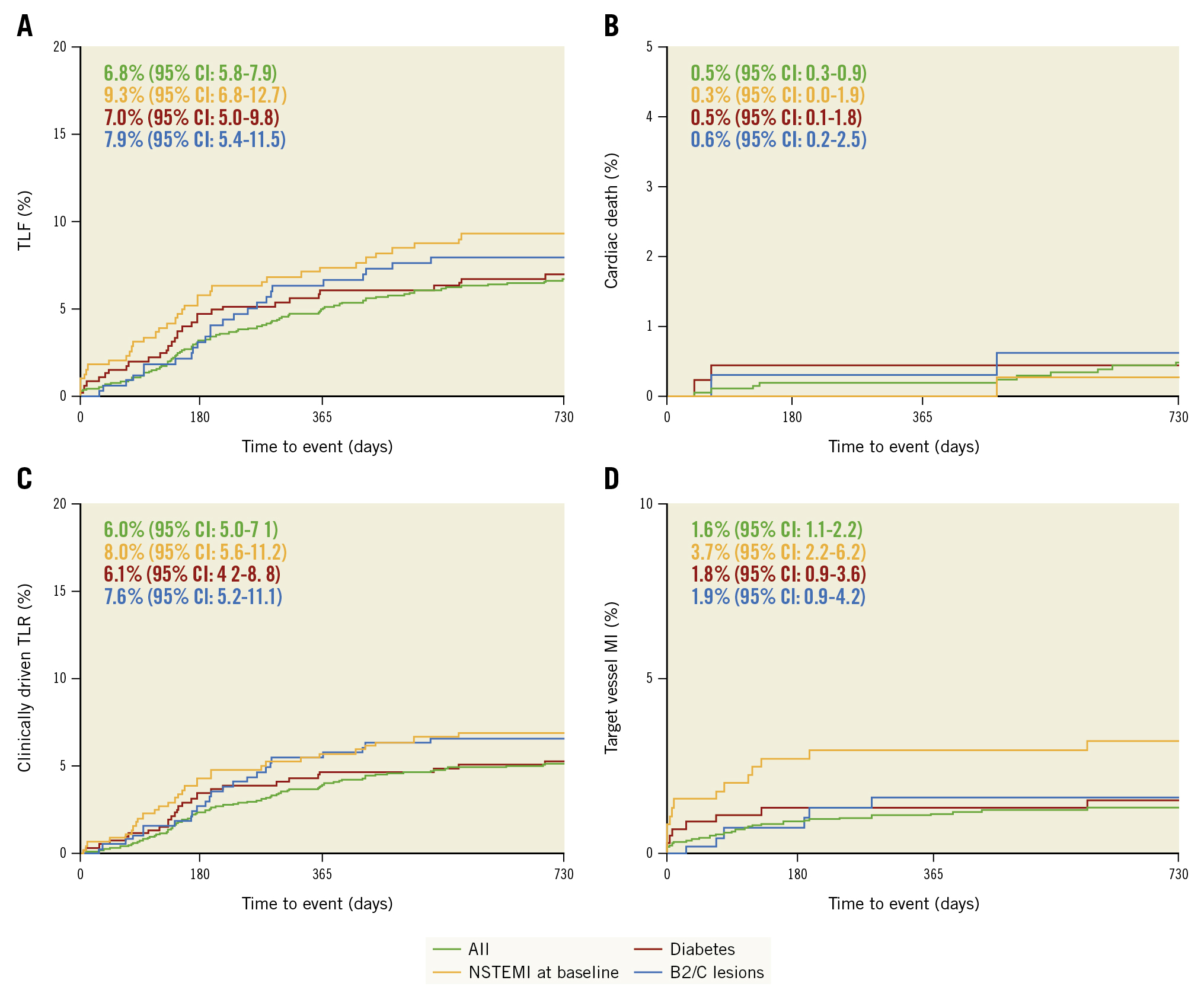
Central illustration. 24-month target lesion failure and its composite for the overall cohort and the subgroup of patients with diabetes, NSTEMI, and type B2/C lesions. Kaplan-Meier estimates in the overall cohort and in high-risk subgroups. A) TLF and its subcomponents cardiac death (B), clinically-driven TLR (C) and target-vessel MI (D). MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; TLF: target lesion failure; TLR: target lesion revascularisation
Table 3. Kaplan-Meier failure estimate for primary and secondary endpoints.
| Full cohort N=2,057* | NSTEMI N=381 |
Diabetes N=444 |
Type B2/C lesions N=316 |
|
|---|---|---|---|---|
| 12 months | ||||
| TLF | 103 (5.0) [4.2-6.1] | 28 (7.4) [5.2-10.5] | 27 (6.1) [4.2-8.8] | 20 (6.3) [4.1-9.6] |
| Cardiac death | 4 (0.2) [0.1-0.5] | 0 | 2 (0.5) [0.1-1.8] | 1 (0.3) [0.0-2.2] |
| Target vessel MI | 28 (1.4) [0.9-2.0] | 13 (3.4) [2.0-5.8] | 7 (1.6) [0.8-3.3] | 6 (1.9) [0.9-4.2] |
| Clinically driven TLR | 93 (4.5) [3.7-5.5] | 25 (6.6) [4.5-9.6] | 24 (5.4) [3.7-8.0] | 20 (6.3) [4.1-9.6] |
| Emergent CABG | 0 | 0 | 0 | 0 |
| All-cause mortality | 14 (0.7) [0.4-1.2] | 2 (0.5) [0.1-2.1] | 4 (0.9) [0.3-2.4] | 2 (0.6) [0.2-2.5] |
| Clinically driven TVR | 102 (5.0) [4.1-6.0] | 28 (7.4) [5.2-10.6] | 25 (5.7) [3.9-8.3] | 20 (6.3) [4.1-9.6] |
| Scaffold thrombosis (definite/probable) | 16 (0.8) [0.5-1.3] | 5 (1.3) [0.5-3.1] | 4 (0.9) [0.3-2.4] | 4 (1.3) [0.5-3.3] |
| 24 months | ||||
| TLF | 138 (6.8) [5.8-7.9] | 35 (9.3) [6.8-12.7] p=0.025 | 31 (7.0) [5.0-9.8] p=0.770 | 25 (7.9) [5.4-11.5] p=0.359 |
| Cardiac death | 10 (0.5) [0.3-0.9] | 1 (0.3) [0.0-1.9] p=0.494 | 2 (0.5) [0.1-1.8] p=0.901 | 2 (0.6) [0.2-2.5] p=0.685 |
| Target vessel MI | 32 (1.6) [1.1-2.2] | 14 (3.7) [2.2-6.2] p=0.0002 | 8 (1.8) [0.9-3.6] p=0.633 | 6 (1.9) [0.9-4.2] p=0.599 |
| Clinically driven TLR | 122 (6.0) [5.0-7.1] | 30 (8.0) [5.6-11.2] p=0.063 | 27 (6.1) [4.2-8.8] p=0.858 | 24 (7.6) [5.2-11.1] p=0.176 |
| Emergent CABG | 0 | 0 | 0 | 0 |
| All-cause mortality | 30 (1.5) [1.0-2.1] | 4 (1.1) [0.4-2.8] p=0.475 | 7 (1.6) [0.8-3.3] p=0.821 | 6 (1.9) [0.9-4.2] p=0.486 |
| Clinically driven TVR | 138 (6.8) [5.8-8.0] | 37 (9.9) [7.3-13.4] p=0.007 | 30 (6.8) [4.8-9.6] p=0.945 | 26 (8.3) [5.7-11.9] p=0.246 |
| Scaffold thrombosis (definite/probable) | 17 (0.8) [0.5-1.3] | 5 (1.3) [0.5-3.1] p=0.242 | 4 (0.9) [0.3-2.4] p=0.841 | 4 (1.3) [0.5-3.3] p=0.351 |
| Data are displayed as n (Kaplan-Meier failure estimates) [95% CI] log-rank. The log-rank test compared the NSTEMI to the non-NSTEMI group, the diabetes to the non-diabetes group, and Type A/B1 lesion group to Type B2/C lesions. Data are patient based. *2,066 patients – 9 patients not implanted (4 patients in the first cohort). CABG: coronary artery bypass graft; CI: confidence interval; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; TLF: target lesion failure; TLR: target lesion revascularisation; TVR: target vessel revascularisation | ||||
Discussion
In this large real-world registry with more than 2,000 patients and endpoints powered for TLF and definite or probable scaffold thrombosis, the sirolimus-eluting Magmaris resorbable scaffold achieved good safety and efficacy outcomes, meeting its prespecified safety and performance criteria. The safety and efficacy were also confirmed in the high-risk subgroups of patients with NSTEMI, diabetes mellitus and type B2/C lesions.
The high device and procedural success rates (97.6% and 99.3%, respectively) despite a high number of first-time users is encouraging and confirms a good conformability and a DES-like behaviour of the Magmaris, which is superior to polymeric scaffolds1213. Furthermore, the high rate of pre- and post-dilatation highlights the adherence to implantation guidelines such as the “4P” strategy8.
Relatively simple lesions were selected, with only 15.3% type B2/C lesions and only 7.5% moderate to severely calcified lesions. However, in contrast to the precursor BIOSOLVE-II and -III studies14, patients with NSTEMI were permitted in BIOSOLVE-IV.
This might have resulted in the slightly higher rate of TLF observed in our series compared to the pooled 12-month outcomes of BIOSOLVE-II and -III (5.0%, 95% CI: 4.1-6.0 vs 3.3%, 95% CI: 1.2-7.1)14, as the NSTEMI subgroup had significantly higher TLF, target vessel MI, and clinically driven TLR rates compared to non-NSTEMI patients. However, as the confidence intervals overlap, this could be a chance finding. Moreover, BIOSOLVE-II and -III were controlled trials with a limited number of centres and with experienced users, whereas in BIOSOLVE-IV, most centres were first-time users, as the registry started immediately after gaining the CE mark. Notably, the TLF rate is still below the expected weighted mean3, and comparable to contemporary DES for which 12-month TLF rates between 4.0% and 10% are reported1516171819. At 24 months, TLF was estimated as 6.8% compared to 5.5% in BIOSOLVE-II and -III1, 7.4% in the Magmaris Italian registry that also included NSTEMI patients, and 5 to 11.9% seen in contemporary DES trials1618192021.
The same applies to the outcomes in terms of definite and probable scaffold thrombosis. While it is disappointing that the “zero scaffold thrombosis” series in BIOSOLVE-II and -III could not be continued in BIOSOLVE-IV, it must be noted that 9 out of 17 scaffold thromboses occurred after the premature discontinuation of antiplatelet or anticoagulation therapy. Furthermore, all but one scaffold thrombosis in the overall cohort occurred between post-procedure days 6 and 188, hence, prior to the resorption time of the Magmaris and thus potentially influenced by Magmaris remnants, which have been observed in optical coherence tomography (OCT) studies22. Notably, the ESC guidelines provide a recommendation of at least 12-month DAPT in patients treated with resorbable scaffolds, up to the presumed time of full resorption2. Another contributing factor could be – as mentioned above for TLF – the large number of operators being first-time users at the beginning of their learning curve. Nevertheless, scaffold thrombosis rates are still within the range for DES151617181921 and lower than those of the Absorb BVS (12-month definite or probable scaffold thrombosis rate of 0.8% vs 1.7% observed in the European ABSORB Consortium registries23).
In terms of subgroups, other series with NSTEMI patients treated with the Magmaris revealed better outcomes. For example, the Magmaris-acute coronary syndrome (ACS) Registry with 65.8% NSTEMI patients revealed a 24-month TLF rate of only 5.2% with absence of scaffold thrombosis, and Magmaris achieved similar outcomes to the Ultimaster (Terumo) DES at 12 months in diabetic patients enrolled in this registry2425. In the Italian registry with 15% NSTEMI patients, 12- and 24-month scaffold thrombosis was 0.5%20; in a series of 75 NSTEMI patients, no scaffold thrombosis or target vessel MI was observed at 6 months22; and in a series of 193 patients with 84.5% NSTEMI patients, no scaffold thrombosis occurred at 12 months26. However, these series mostly involved a limited number of mostly experienced centres, which could have impacted outcomes.
It is encouraging that the clinical outcomes do not differ significantly between diabetic and non-diabetic patients or type B2/C and type A/B1 lesions, albeit these were post hoc analyses and might lack statistical power. Likewise, there was no relevant difference between diabetic and non-diabetic patients in other series24. Furthermore, beyond 12 months, the TLF curve starts to flatten.
There are still many unknowns in terms of implantation techniques; for example, recent findings indicate that performing repetitive post-dilatation to optimise the lumen area and reduce malapposition may compromise the mechanical integrity of the scaffold25. Furthermore, the late lumen loss assessed in BIOSOLVE-II and -III14 and the TLR rates observed in BIOSOLVE-IV still bear room for improvement. It remains to be seen if the third-generation drug-eluting resorbable magnesium scaffolds (DREAMS 3G; BIOTRONIK) will provide improved performance outcomes while maintaining the safety profile of the Magmaris. DREAMS 3G is currently being tested in the BIOMAG-I study (ClinicalTrials.gov: NCT04157153).
Limitations
The main limitation of the BIOSOLVE-IV registry is the single-arm design, hampering comparisons to other devices. However, the aim of BIOSOLVE-IV was not to compare it against other devices, but to ensure a safe rollout of this new technology. As is inherent to the nature of an observational registry, angiographic outcomes were not assessed. Additionally, the use of IVUS and OCT was not recorded. The strengths of BIOSOLVE-IV are the good follow-up compliance and that events were adjudicated by an independent clinical events committee.
Conclusions
The BIOSOLVE-IV registry showed good safety and performance outcomes, which were comparable to contemporary DES, confirming its safe rollout into clinical practice. In contrast to precursor studies, scaffold thromboses were observed, emphasising the need for strict antiplatelet therapy adherence. However, scaffold thrombosis rates are still low and lower than observed for first-generation resorbable polymeric scaffolds, particularly in terms of late thrombotic events.
Impact on daily practice
This is the largest series of 24-month outcomes for the second-generation drug-eluting absorbable magnesium scaffold to date (DREAMS 2G, commercial name Magmaris), powered for the endpoints of definite or probable scaffold thrombosis and TLF. The performance objectives were met, confirming the safe rollout into clinical practice after obtaining market approval in Europe and Asia with good safety and performance outcomes comparable to state-of-the-art DES; however, the importance of strict DAPT adherence was highlighted, as half of the scaffold thromboses occurred after premature DAPT interruption. With more than 2,000 patients enrolled, BIOSOLVE-IV addresses the call for more data from the European medical societies.
Acknowledgments
We thank Beatrix Doerr, Consultant Medical Writer, for her help in preparing this manuscript; she was reimbursed by BIOTRONIK.
Funding
The study was funded by BIOTRONIK. The sponsor was involved in the design, conduct and analysis of the study, and reimbursed the medical writer that helped to prepare the manuscript.
Conflict of interest statement
J. Torzewski reports speaker fees and support to attend EuroPCR 2022 from BIOTRONIK; and is an associated editor of Frontiers in Cardiovascular Medicine. J. Bennett has received research grants and speaking fees from Abbott Vascular and BIOTRONIK. M. Haude reports grants and/or contracts from BIOTRONIK, Cardiac Dimensions, OrbusNeich, and Philips; consulting fees from BIOTRONIK, Cardiac Dimensions, and OrbusNeich; honoraria and/or speaker fees from BIOTRONIK, Cardiac Dimensions, OrbusNeich, and Philips; support to attend meetings/travel support from BIOTRONIK; is a steering committee member of the BIOSOLVE and BIOMAG trials; and is a past president of EAPCI. M. Wiemer reports lecturer honoraria from BIOTRONIK and received support from BIOTRONIK for attending EuroPCR 2022. S. Verheye reports grants/contracts from BIOTRONIK and Elixir Medical; consulting fees from Neovasc; and speaker fees from Elixir Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

