
Abstract
Aims: The second-generation drug-eluting absorbable magnesium scaffold Magmaris, recently introduced for the treatment of obstructive coronary atherosclerotic lesions, suggests a good safety profile, but preclinical assessment is important for predicting clinical performance. The aim of the present study was to assess subacute and long-term safety as well as pharmacokinetic properties of the Magmaris compared with a current-generation metallic DES and an approved BRS in porcine and rabbit animal models.
Methods and results: Ninety Magmaris scaffolds were implanted into non-diseased porcine and rabbit models. A bioresorbable vascular scaffold (Absorb) and a permanent drug-eluting stent (XIENCE Xpedition) served as controls. Scanning electron microscopy showed increased endothelialisation and decreased thrombus formation at three and 28 days in the Magmaris group compared with the Absorb group. In the XIENCE group, inflammation exceeded the level in the Magmaris group at 365 and 730 days. Neointimal growth was greater in the Magmaris group than in the XIENCE group. Late lumen loss decreased over time in both groups. Optical coherence tomography (OCT) showed stable luminal dimensions in both the Magmaris and XIENCE groups. Pharmacokinetic studies demonstrated a retarded elution profile in the Magmaris group with 69.4% of sirolimus released at 90 days.
Conclusions: Preclinical results suggest that the Magmaris has a favourable safety profile with advanced healing relative to benchmark, low acute thrombogenicity, and absence of excessive lumen loss up to two years. These results support clinical application of Magmaris for human use.
Abbreviations
ANOVA: analysis of variance
AUC0-inf: area under the curve from zero to infinity
BRS: bioresorbable vascular scaffolds
Cmax: maximum blood concentration
DES: drug-eluting stents
DREAMS: drug-eluting absorbable metal scaffold
EEL: external elastic laminae
EES: everolimus-eluting stent
HPLC-MS/MS high-performance liquid chromatography-tandem mass spectrometry
IEL: internal elastic laminae
LLI: late loss index
LLL: late lumen loss
OCT: optical coherence tomography
PDLLA: poly D,L-lactic acid
PLLA: poly-L-lactic acid
QCA: quantitative coronary angiography
SEM: scanning election microscopy
Tlast: time point of last quantifiable concentration
Introduction
Bioresorbable vascular scaffolds (BRS) represent a disruptive technology in the treatment of obstructive atherosclerotic coronary lesions because of their temporary presence, which permits vascular restoration over time. The polymer-based BRS Absorb (Abbott Vascular, Santa Clara, CA, USA) received US Food and Drug Administration approval based on the prospective randomised multicentre trial ABSORB. The study showed non-inferiority to metallic everolimus-eluting stents1. The main hypothetical advantage of BRS relates to their temporary presence. During scaffold degradation, vasomotion is gradually restored as a function of time and loss in radial strength. Despite the early favourable results achieved with BRS, important limitations became apparent, including inferior deliverability and radial force and higher rates of early and late scaffold thrombosis compared with the latest generation of metallic drug-eluting stents (DES)2-6. Metallic bioresorbable scaffolds have been introduced as a viable option to overcome some of the limitations of first-generation BRS technology. The second-generation drug-eluting absorbable metal scaffold (DREAMS) Magmaris™ (Biotronik, Bülach, Switzerland) showed a good safety profile in clinical tests with absence of definite or probable scaffold thrombosis7.
The aim of the present study was to assess subacute and long-term safety as well as the pharmacokinetic properties of the Magmaris at up to 24 months compared with a current-generation metallic DES (XIENCE; Abbott Vascular) and an approved BRS (Absorb; Abbott Vascular) in porcine and rabbit animal models.
Methods
STUDY DEVICE AND CONTROLS
The test device Magmaris is a drug-eluting absorbable metal scaffold consisting of a 3.0×20 mm magnesium alloy backbone with six crown two link square-shaped struts at a thickness and width of 150/150 μm that can be expanded safely up to 0.6 mm above the nominal scaffold diameter. The BIOlute® coating (Biotronik) is composed of a bioresorbable poly-L-lactic acid (PLLA) polymer matrix loaded with sirolimus (140 µg/cm2). For subacute and 28-day endothelialisation studies, the Absorb bioresorbable vascular scaffold, consisting of a 3.0×18 mm polymer backbone of PLLA coated with a 1:1 blend of everolimus (100 µg/cm2) and poly (D,L-lactide) (PDLLA) with a strut thickness and width of 150/180 μm, was used as control. For the long-term safety study, the XIENCE Xpedition 3.0×18 mm (Abbott Vascular), a cobalt-chromium stent with the same drug density as Absorb (100 µg/cm2) and a strut thickness and width of 81/100 μm, was used as control.
ANIMAL MODELS
A juvenile porcine coronary stent model was applied to assess subacute and long-term safety and pharmacokinetics (n=90 pigs). Endothelialisation was investigated in a healthy rabbit model of iliofemoral stent implantation (n=8 animals) (Figure 1).
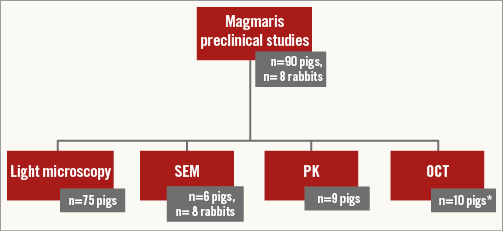
Figure 1. Study flow chart. *n=10 pigs with combined light microscopy and OCT. OCT: optical coherence tomography; PK: pharmacokinetic studies; SEM: scanning electron microscopy
SUBACUTE AND LONG-TERM SAFETY EVALUATION IN A PORCINE MODEL
The early healing response at three days after implantation of the Magmaris was compared to Absorb based on evaluation of platelet-fibrin surface thrombi, inflammatory cell response and endothelial cell coverage in six hybrid farm pigs using scanning electron microscopy (SEM) analysis.
Long-term safety with regard to inflammation and neointimal growth of the Magmaris was compared with XIENCE at one, three, six, 12, and 24 months using angiography, optical coherence tomography, histomorphometry, and histopathology data acquired in 75 non-atherosclerotic Yucatan mini swine.
Study protocols were approved by the Institutional Animal Care and Use Committee of the testing facility (AccelLAB Inc., Boisbriand, Quebec, Canada) and were in compliance with the Canadian Council on Animal Care regulations. Animal husbandry, medication administration, and stent implantation were performed according to standards as previously reported.
HARVEST OF SAMPLES AND HISTOPATHOLOGY
In the subacute endothelialisation study, six coronary arteries treated with the Magmaris and six with the Absorb were harvested three days after implantation into hybrid farm pigs. All implanted arteries were fixed in situ with neutral buffered formalin after perfusion with Ringer’s lactate to remove blood. Treated arteries were stored in glycerol until preparation for SEM was initiated.
Long-term treated vessel sections were harvested at four weeks (19 animals; 12 Magmaris/12 XIENCE), 12 weeks (22; 12/12), 25 weeks (19; 12/11), one year (7; 8/8), and two years (8; 10/9).
Neointimal inflammation (0-4, 0=<25% struts with fewer than 10 inflammatory cells, 1=up to 25% struts with more than 10 inflammatory cells, 2=25-50% struts with more than 10 inflammatory cells, 3=>50% struts with more than 10 inflammatory cells, 4=two or more struts associated with granulomatous inflammatory reactions) and fibrin (0-3) were semi-quantitatively scored for each section. A vessel injury score was calculated according to the Schwartz method8.
QCA AND MORPHOMETRY
Quantitative coronary angiography (QCA) and histomorphometry analyses were performed as described previously9. QCA was performed with Medis QCA-CMS 6.0 software (Medis, Leiden, the Netherlands). For the histological analysis by light microscopy with image capture, ~8-μm-thick histological sections of the proximal, middle, and distal segments of the stented artery were stained with haematoxylin-eosin and Verhoeff-van Gieson stain. Morphometry was performed using Image-Pro Plus 6.1.0.346 software (Media Cybernetics Inc., Silver Spring, MD, USA).
OCT
Optical coherence tomography was performed in 6/4/4 Magmaris and 2/2/2 XIENCE treated vessels of the 180 d/1 yr/2 yr cohorts, respectively, after implantation and before sacrifice with the ILUMIEN™ C7 imaging system (St. Jude Medical, St. Paul, MN, USA). Nitroglycerine was administered to achieve vasodilatation and the OCT catheter was advanced beyond the device into the distal non-stented vascular segment. The pullback covered the distal non-stented, stented and proximal non-stented arterial segments.
SEM ANALYSIS OF ENDOTHELIALISATION AND THROMBUS FORMATION IN A RABBIT MODEL
Eight adult female and male New Zealand White rabbits (3.3-3.6 kg) underwent endothelial denudation of both iliac arteries using a balloon catheter. Subsequently, Magmaris and Absorb scaffolds were deployed in one of the iliac arteries of each animal at the site of denudation, resulting in a total of 16 implants with a target device-to-artery ratio of 1.3:1. For Magmaris, the balloon was inflated for at least 10 seconds. Absorb was implanted according to IFU. Following device implantation, final angiograms of the treated vessel were performed and the peripheral arterial flow was evaluated. Animals were treated with approximately 40 mg of acetylsalicylic acid and approximately 25 mg of clopidogrel daily given per os in an appropriate carrier.
The segments of the three main coronary arteries were assigned to different stents using a predetermined stratified matrix. The implanted arteries, treated with eight Magmaris and eight Absorb devices, were harvested 28 days after implantation.
All implanted arteries were fixed in situ with neutral buffered formalin after perfusion with Ringer’s lactate to remove blood. Treated arteries were stored in glycerol until preparation for SEM was initiated. SEM imaging and analysis was performed at CVPath Institute (Gaithersburg, MD, USA), as previously reported.
Low power images at 15x magnification were acquired of the lumen surface to estimate the degree of endothelial coverage visually. Regions of interest were photographed at incremental magnifications of 50x, 200x, and 600x. From these images, the percentage of endothelial coverage was visually assessed between and above scaffold struts; findings regarding platelet-fibrin surface thrombi, and inflammatory cell response were qualitatively assessed as either absent or present.
EVALUATION OF MAGMARIS PHARMACOKINETICS IN A PORCINE MODEL
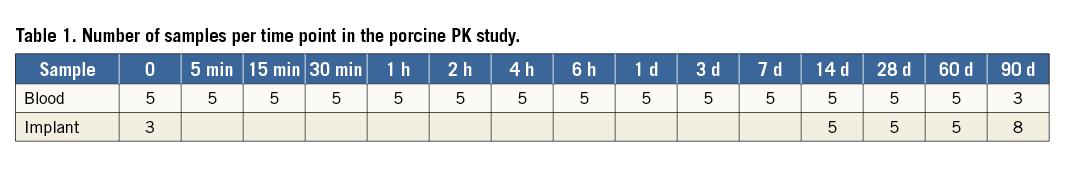
Nine hybrid farm pigs were allocated to pharmacokinetic studies using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) to characterise release kinetics as well as blood and tissue concentration of sirolimus up to 90 days. A total of 73 blood samples from three to five different animals per time point were analysed to allow calculation of the mean values at baseline, five, 15, 30 min, one, two, four and six hours, and one, three, seven, 14, 28, 60 and 90 days.
To evaluate release kinetics and assess drug uptake into tissue, 23 scaffolds from five to eight different animals per time point were explanted; scaffold and tissue material were separated under microscopic guidance and analysed for remaining drug on the device as well as drug concentration in tissue at 14, 28, 60, 90 days (Table 1). In addition, three naive control devices were measured as baseline control.
STATISTICAL METHODS
Histopathology and QCA variables were first checked for normal distribution using the Lilliefors corrected Kolmogorov-Smirnov test and then separated into variables with normal and non-normal distribution. Mean values with standard deviation were derived from normally distributed parameters while non-normally distributed data were described as median with 25% and 75% percentiles. The Kruskal-Wallis test with Dunnett’s post hoc correction was used for comparison of non-normally distributed data. In the event of normal distribution, variables were compared using the Student’s t-test or ANOVA with Dunnett’s post hoc correction for multiple comparisons, when applicable. To derive mean differences between baseline and follow-up OCT measurements, a paired Wilcoxon signed-rank test was applied. A value of p≤0.05 was considered statistically significant.
For the 3D subacute data, initial equal variance tests (Levene) and normality test (Kolmogorov-Smirnov) were performed. If both were successful, ANOVA was performed. If either variance or normality tests failed, a Kruskal-Wallis test with Dunn’s post hoc correction was performed.
For 28-day endothelialisation data, initial equal variance tests (Levene) and normality test (Kolmogorov-Smirnov) were performed. If both were successful, t-tests were used to compare group means. If either variance or normality tests failed, a Mann-Whitney (aka Wilcoxon rank-sum) test was performed.
Results
SUBACUTE SAFETY AT THREE DAYS
Scanning electron microscopic evaluation of Magmaris and Absorb after three days in hybrid farm pig coronary vessels revealed a wide and even expansion of implants with struts well apposed to the vessel wall and no evidence of occlusive thrombi associated with either of the tested scaffolds.
In comparison to Absorb, Magmaris was associated with a higher degree of endothelialisation above struts (Magmaris 30.5±9.4% vs. Absorb 6.8±1.8%), between struts (Magmaris 63.5±5.9% vs. Absorb 56.0±17.6%), as well as overall endothelialisation (Magmaris 47.0±4.1% vs. Absorb 31.4±9.2%) (Table 2, Figure 2A). Absorb showed a greater proportion of thrombi overlying struts and extending into the luminal surface between the struts as compared with Magmaris (Figure 2B, Figure 2C).
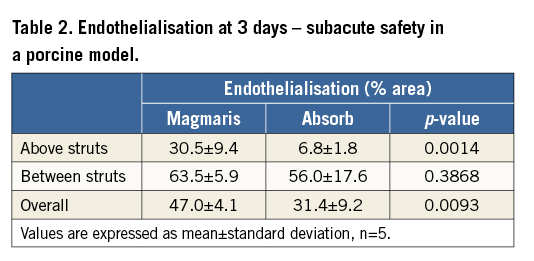
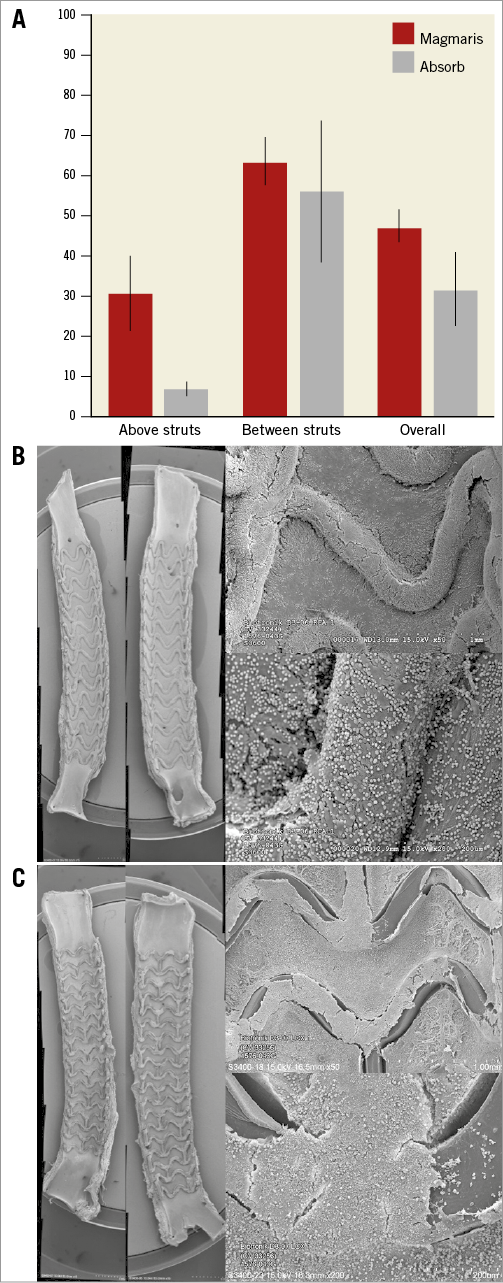
Figure 2. Subacute safety in a porcine model. Endothelialisation after three days (A) as evaluated in SEM for Magmaris (B) and Absorb (C). Representative low (x15, bisected vessel, far left) and high power (x50, upper right, x200, lower right) SEM images. Values are expressed as mean±standard deviation.
ENDOTHELIALISATION AT 28 DAYS
After 28 days in rabbit iliac arteries, Magmaris showed greater endothelialisation above struts compared to Absorb (51.7±21.2% and 19.3±16.0%, respectively) (Table 3, Figure 3), as well as overall endothelialisation (73.8±10.5% and 59.2±8.0%, respectively) in comparison to Absorb. Neither scaffold type showed significant thrombus deposition on the strut surfaces at this time point.
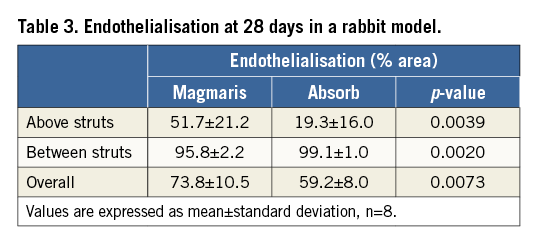
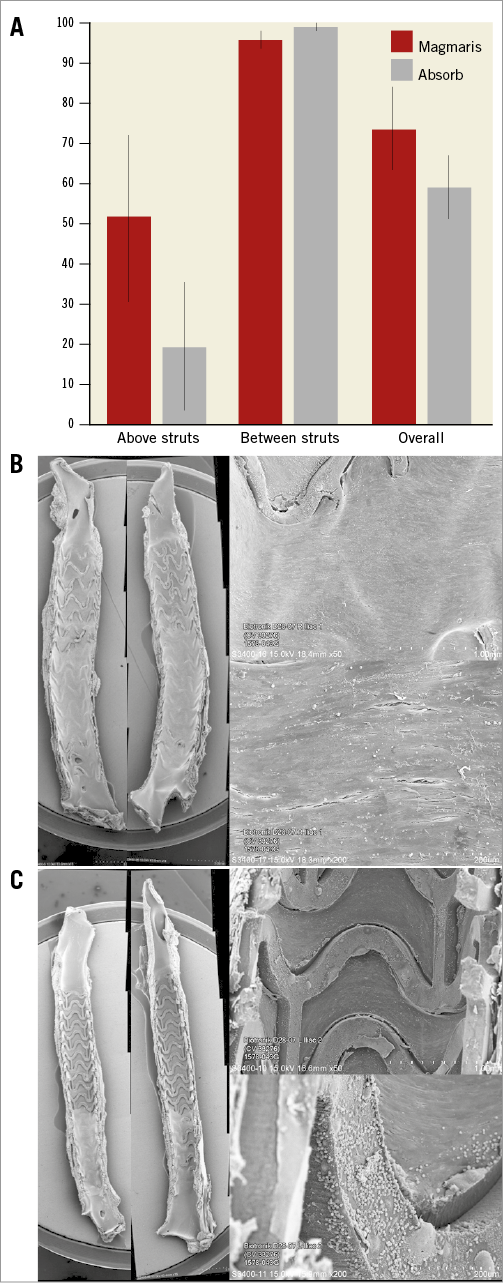
Figure 3. Endothelialisation after 28 days. Endothelialisation in rabbit iliac arteries (A) as evaluated by SEM for Magmaris (B) and Absorb (C). Representative low (x15, bisected vessel, far left) and high power (x50, upper right, x200, lower right) SEM images at 28 days. Values are expressed as mean±standard deviation.
QCA, HISTOPATHOLOGY AND OPTICAL COHERENCE TOMOGRAPHY
QCA confirmed similar balloon-to-artery ratio for both devices at all time points. At 28, 90, and 180 days, mild lumen narrowing was observed in both groups with greater lumen loss (LLL) and diameter stenosis in vessels treated with Magmaris compared to XIENCE (Table 4). Peak LLL and diameter stenosis were detected at three months for both Magmaris and XIENCE. LLL was similar among Magmaris and XIENCE at 360 days, and turned into late lumen gain after two years, which was greater in Magmaris- as compared to XIENCE-treated arteries (Figure 4).
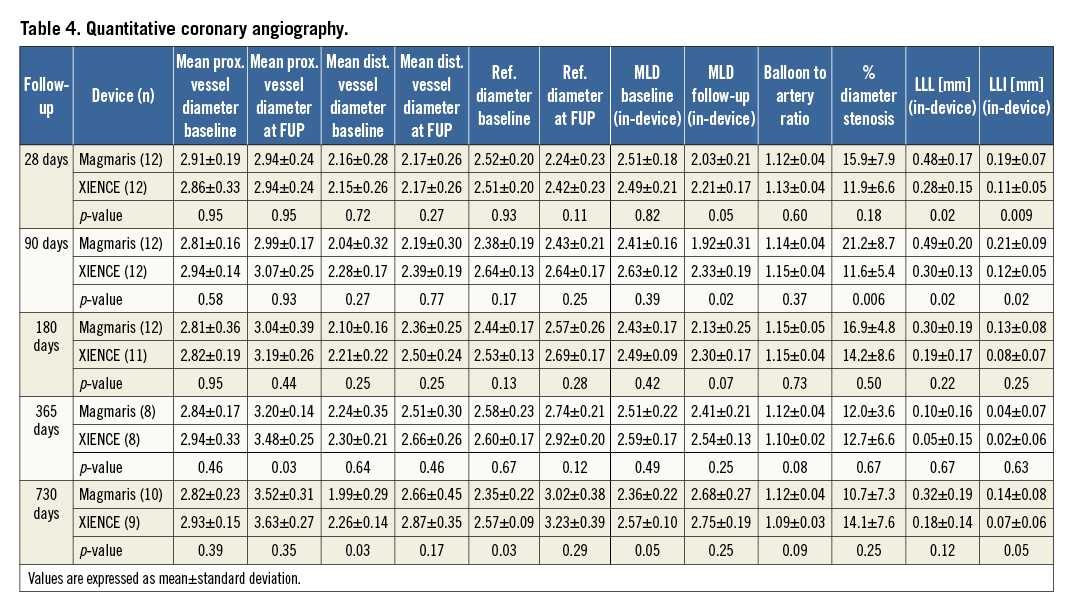
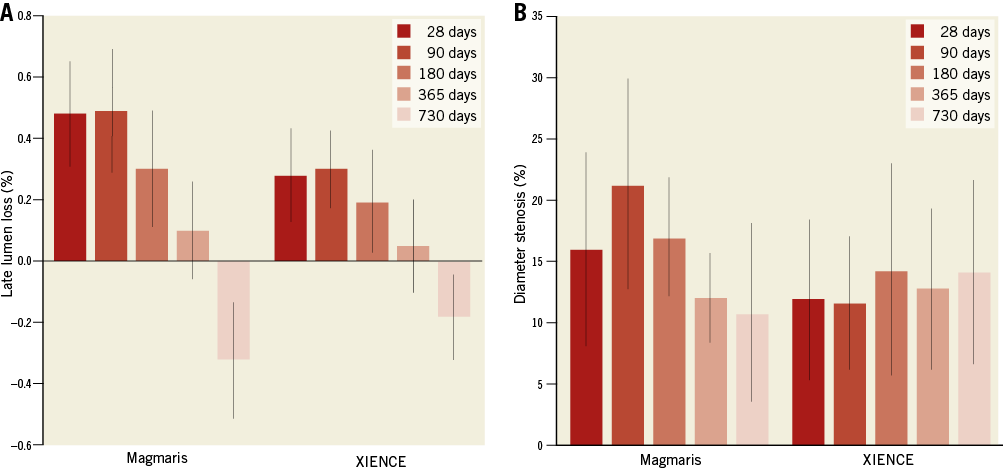
Figure 4. Late lumen loss and percentage diameter stenosis in a non-atherosclerotic porcine model. A) Late lumen loss. B) Percentage diameter stenosis. Values are expressed as mean±standard deviation.
Neointimal area was greater in Magmaris as compared to XIENCE at all time points and remained stable up to 730 days (2.25 [1.95-2.50] vs. 1.45 [1.29-1.80], p=0.0009) in Magmaris compared with XIENCE (Table 5, Figure 5). Inflammation score of Magmaris peaked at 90 days (1.67 [0.00-3.75]) and subsequently decreased up to 730 days (1.00 [0.67-1.42]). In contrast, XIENCE showed minimal to absent inflammation up to 90 days and subsequently increased with a peak at 365 days (1.83 [0.75-2.00]), which was greater than Magmaris at this time point. Granulomatous reactions were observed in both treatment groups and peaked at 90 days, where Magmaris showed a significantly greater percentage of stent struts with granulomatous reactions (8.33 [0-83.8] for Magmaris vs. 0 for XIENCE, p=0.04). At 365 and 730 days, granulomatous reactions were almost absent in both groups. Giant cell reactions were maximum between 90 and 180 days and remained higher in Magmaris compared to XIENCE up to 730 days. Fibrin deposition was moderate in all Magmaris- and XIENCE-treated vessels at 28 days and very low at all time points thereafter. Injury score was greater in Magmaris compared with XIENCE at all time points; however, statistical significance was only reached at 28 and 90 days (Table 5). Lumen area by OCT increased at follow-up in both Magmaris and XIENCE between 180 and 730 days, with a greater absolute increase in Magmaris compared with XIENCE. Furthermore, the decrease in lumen area between baseline and follow-up imaging was significantly less in Magmaris compared with XIENCE at 730 days (Table 6, Figure 6).
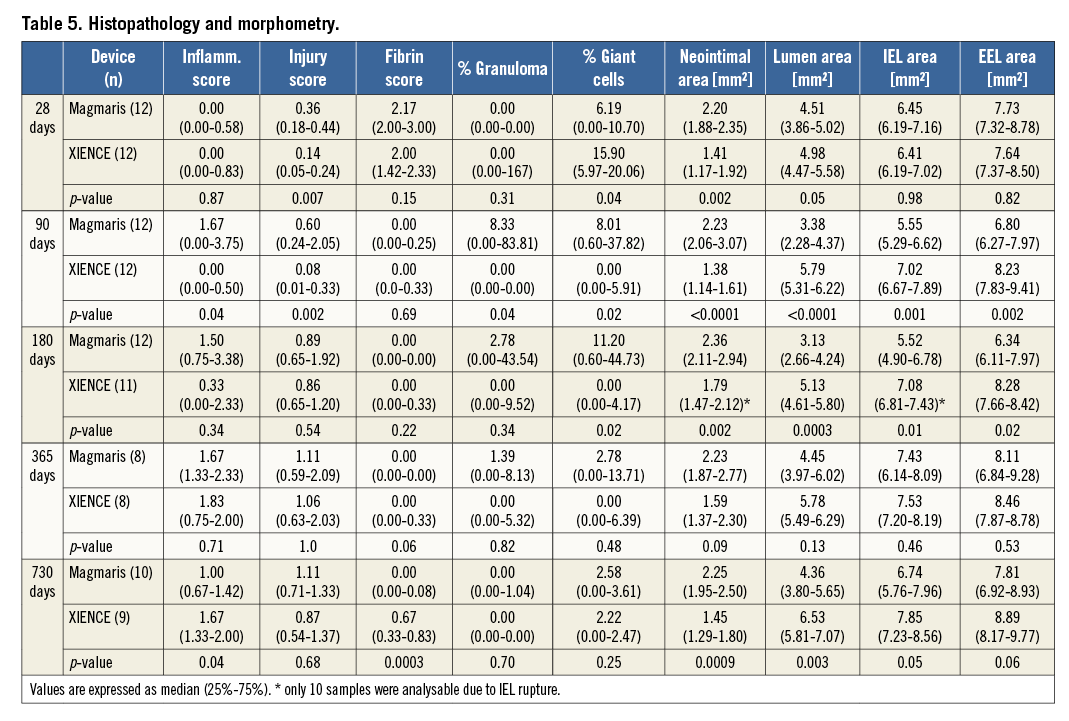
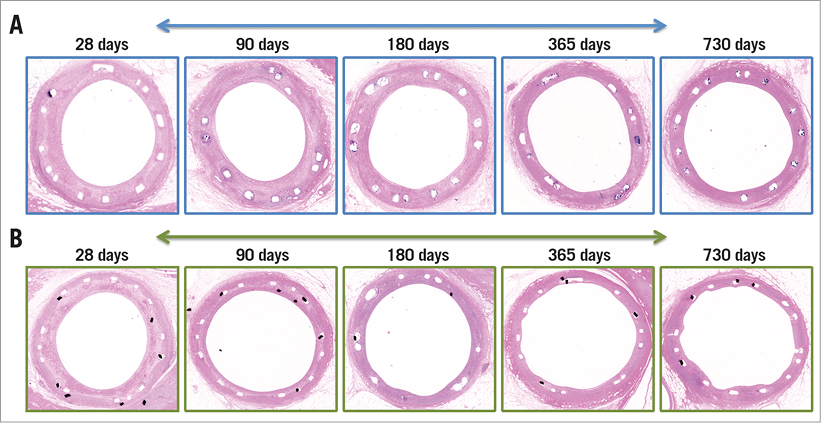
Figure 5. Representative H&E cross-sections. Vessels treated with Magmaris (A) and XIENCE (B) at all time points.
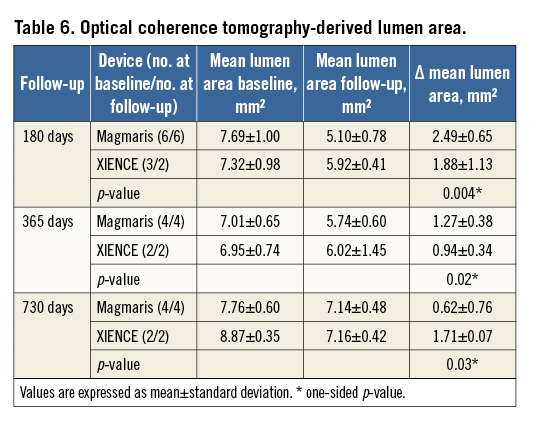
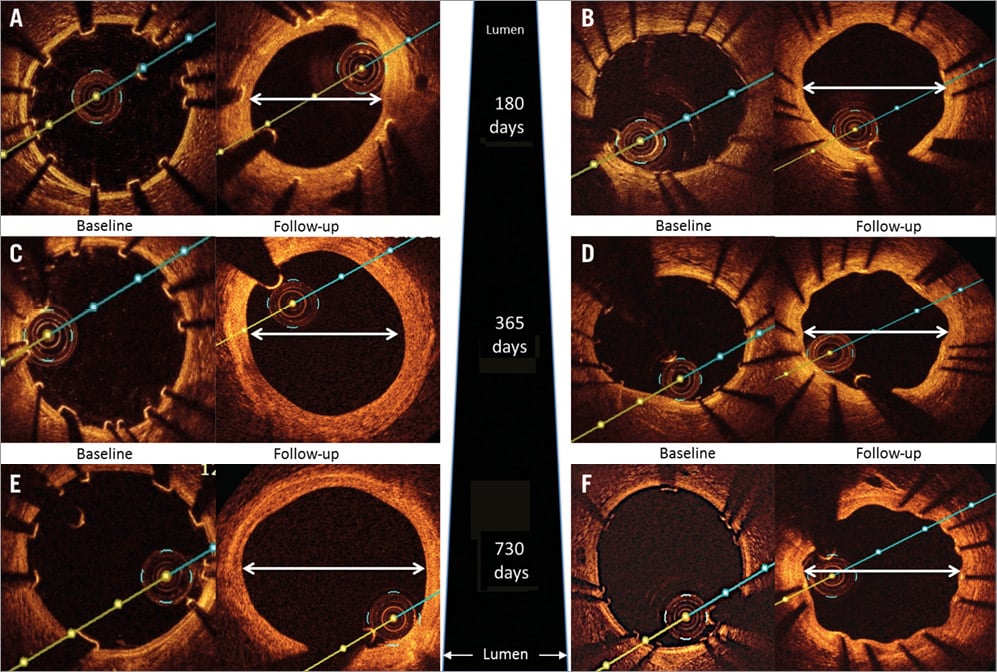
Figure 6. OCT images at different time points. OCT images of Magmaris (A,C,E) and XIENCE (B,D,F) at different time points after implantation into porcine coronary arteries. Post implantation, the device is clearly visible with well-apposed struts with decreasing visibility of the scaffold struts and absence of excessive lumen loss over time as seen at 180 days (A,B), one year (C,D) and two years (E,F) after implantation.
PRECLINICAL EVALUATION OF MAGMARIS PHARMACOKINETICS
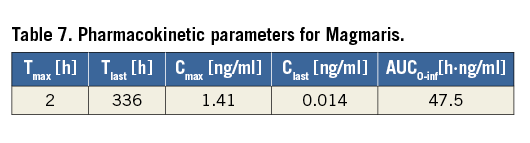
At two hours after implantation of Magmaris, a peak concentration (Cmax) of sirolimus in the blood of 1.405±0.262 ng/ml was observed. Thereafter, the sirolimus blood levels quickly decreased with a time point of last quantifiable concentration of 0.014 ng/ml (Tlast) at 14 days. Pharmacokinetic analysis revealed a systemic drug exposure (AUC0-inf) of 47.5 h*ng/ml per scaffold (Table 7).
The mean cumulative sirolimus release was 69.4% at 90 days, with 56.7% released at 28 days (Figure 7).
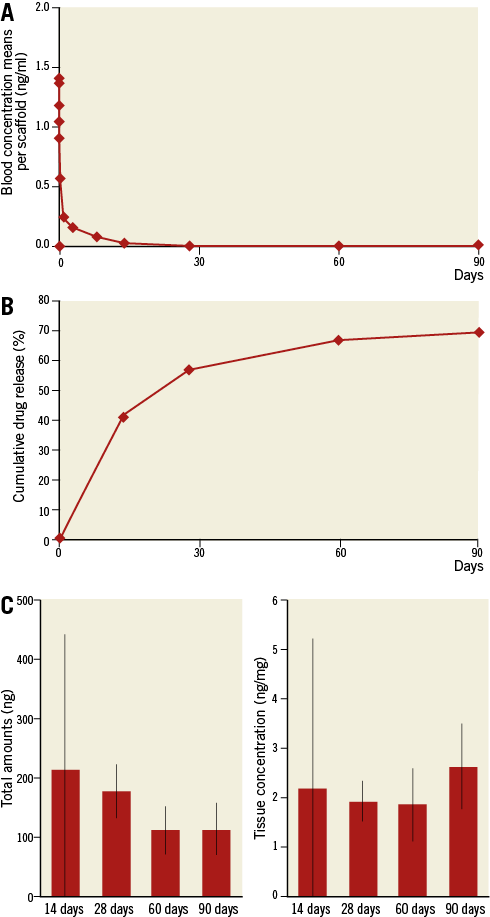
Figure 7. Sirolimus concentration in whole blood, in vivo cumulative drug release, and drug amounts/concentrations in treated artery segments. A) Sirolimus concentration in whole blood. B) Cumulative drug release. C) Drug amounts/concentrations. Values are expressed as mean±standard deviation.
Drug uptake in treated coronary vessel sections revealed a peak mean total amount at 14 days (213.90 ng) and presence of drug (2.62 ng/mg) up to 90 days (Figure 7).
Discussion
The salient findings of these results from two preclinical animal models are:
Acute thrombogenicity in coronary arteries was significantly lower in Magmaris relative to Absorb implanted in juvenile hybrid farm pigs for three days, which can be explained by higher re-endothelialisation in Magmaris as compared to Absorb. Improved endothelialisation of Magmaris was further confirmed in rabbit iliac arteries at 28 days.
Vascular compatibility, including neointimal growth, inflammation, and fibrin deposition, was similar with Magmaris and XIENCE up to two years of follow-up. While inflammation peaked at 90 days in Magmaris and decreased thereafter, XIENCE showed very low inflammation until 90 days and subsequently increased over time. Neointimal growth was greater in Magmaris as compared to XIENCE at all time points.
Angiographic surveillance revealed moderate late lumen loss in Magmaris up to 365 days, which was mitigated by some lumen gain at 730 days. Imaging analysis by OCT confirmed absence of excessive lumen loss up to 730 days in both Magmaris and XIENCE.
Pharmacokinetic analysis of tissue and blood revealed peak tissue levels of sirolimus at 14 days after release of 41.0% of the drug, with blood levels of sirolimus below the lower limit of detection after 14 days.
ACUTE COMPARATIVE THROMBOGENICITY AND ENDOTHELIALISATION
Increased acute thrombogenicity of Absorb BRS relative to contemporary DES has recently been shown to be a nidus of thrombus formation in a porcine arteriovenous shunt model10 and in post-marketing studies11,12. The in vivo results of the current study confirm the relevance of flow dynamics for thrombogenicity in the acute setting. In this regard, Magmaris showed a favourable outcome, probably explained by the haemodynamically advantageous design, round strut edges due to electropolishing, and the biocompatible BIOlute® coating. In addition, Magmaris had more endothelialisation coverage and, most importantly, greater coverage of stent struts with endothelial cells when compared with Absorb after 28 days in rabbit iliac arteries.
INFLAMMATION AND NEOINTIMAL GROWTH
Inflammation peaked at 90 days in Magmaris, where moderate granulomatous reactions were associated with degrading stent struts. After this time point, inflammatory reactions decreased to minimal at 730 days. In contrast, inflammation was minimal to absent in XIENCE up to 90 days and subsequently increased up to 730 days. While inflammatory reactions to XIENCE V® (Abbott Vascular) did not show late increase in a previous study comparing it against Absorb BRS, strain or breed-related reactions are likely to explain the discrepant results. Although increased inflammation was observed in Magmaris at 90 days, neointimal growth remained stable beyond this up to 730 days. Importantly, the pharmacokinetic drug release profile and tissue concentrations of sirolimus confirmed the presence of antiproliferative drug beyond the critical 90-day time point, which might explain the consistency in neointimal growth up to 730 days. The delay in inflammation of the Absorb BRS relative to Magmaris is explained by the faster pace of degradation in the latter (12 to 18 months in Magmaris vs. 36 to 42 months in Absorb BRS).
LUMEN DIMENSIONS BY ANGIOGRAPHY AND OCT
Angiographic surveillance revealed moderate late lumen loss in Magmaris up to 90 days, which mitigated after this time point and turned into late lumen gain at 730 days. This could be explained by positive remodelling after complete degradation. However, with the absence of lumen growth revealed by OCT, we should be careful not to overinterpret these findings. Surprisingly, XIENCE-treated arteries showed a similar trend of late lumen gain, which may be explained by animal growth over time or potential angiographic variations owing to limited spatial resolution of two-dimensional angiographic assessment. Consecutive OCT imaging between index procedure and follow-up showed a significantly greater decrease in mean lumen area in Magmaris as compared with XIENCE at 180 days, which reversed at 730 days, probably demonstrating greater compensatory vessel growth in the latter, suggestive of vascular restoration.
Study limitations
The present study has several limitations, including the lack of blinding of the study groups to the analysts in the core laboratory due to the nature and visibility of the device compared with metallic stents or other polymeric scaffolds. The study was performed in normal porcine coronaries and cannot take into account the effect of atherosclerosis on degradation and response to Magmaris. Nevertheless, serial follow-up provides mechanistic insight into the biological processes of Magmaris degradation compared with both permanent metallic DES and polymer-based absorbable vascular scaffolds.
Conclusions
These preclinical studies support the efficacy and safety profile of the second-generation drug-eluting magnesium scaffold Magmaris with regard to vascular healing, thrombogenicity, and vascular restoration over two years. Therefore, Magmaris could be utilised as an alternative to metallic DES or Absorb for clinical use.
| Impact on daily practice Magmaris received the CE mark in June 2016 and is now available commercially in Europe. The preclinical results support the safety and the efficacy of the device. |
Acknowledgements
The authors would like to thank the team at AccelLAB which conducted the preclinical studies.
Funding
The study was funded by Biotronik AG (Bülach, Switzerland).
Conflict of interest statement
R. Waksman is a consultant for Amgen, Abbott Vascular, Biosensors International, Biotronik, Boston Scientific, Medtronic Vascular, Symetis, and Lifetech, and serves on the speakers bureau of Abbott Vascular, Amgen, AstraZeneca, and Biotronik. P. Zumstein and M. Pritsch are employees of Biotronik. E. Wittchow is an employee of QualiMed Innovative Medizinprodukte GmbH. M. Haude has received institutional grant/research support from Abbott Vascular, Biotronik, Cardiac Dimensions, Lilly, Medtronic, and Volcano, and is a consultant for Biotronik and Cardiac Dimensions. M. Joner is a consultant for AUM Medical, and Biotronik, and serves on the speakers bureau of Abbott Vascular, AstraZeneca, Biotronik, Boston Scientific, Medtronic, and OrbusNeich. The other authors have no conflicts of interest to declare.

