
Concept and design of a bioresorbable scaffold, which “does its job and disappears”
A fully bioresorbable scaffold (BRS) should be viewed as a transient coronary implantation device which prevents acute recoil and constrictive remodelling for a limited duration of time, elutes an antiproliferative drug to avoid excessive neointimal hyperplasia and then disappears by biodegradation (“does its job and disappears”)1. Since the peak of chronic vascular constriction and neointima hyperplasia occurs approximately three to six months after balloon angioplasty and/or bare metal stenting2,3,4, a BRS providing mechanical support for three to six months with drug elution has the potential to resolve the dilemma between transient therapeutic need and permanent implant of a foreign body; the technology has therefore attracted the interest of inventors, interventionalists and patients1.
In the design of a BRS, it is of importance to programme the timing of the bioresorption, the loss of mechanical integrity, the complete bioresorption and healing; all these timings are controlled by selection and post-processing of materials as well as by the biological reaction of the vessel wall. A variety of bioresorbable materials is available to construct a BRS, including bioresorbable polymer (e.g., poly-D,L-lactide, poly-L-lactide, poly-lactic-co-glycolic acid, polycaprolactone and tyrosine polycarbonate) and biodegradable metals (e.g., magnesium and iron). Each material has a unique mechanism of biodegradation (e.g., hydrolysis, cell-mediated degradation or oxidation). Compared to permanent metals such as cobalt chromium or stainless steel, polymeric bioresorbable materials have an inherent weakness in their mechanical properties, which has to be compensated for by making the struts thicker and wider. Technically speaking, the mechanical properties and biodegradation time of bioresorption materials are modifiable and tunable. For example, a PLLA scaffold could gain a higher mechanical strength by post-processing the PLLA material with longitudinal and radial extrusion, or by increasing the initial molecular weight of the polymer5,6. Although hydrolysis is a purely chemical process, materials bioresorbed by cellular-mediated reaction (Xeltis; Xeltis AG, Zurich, Switzerland) could be influenced by the plaque morphology and its cellular components. The versatility of the bioresorbable materials precludes a “class effect” among the diverse BRS, in acute performance, biodegradation profile and long-term outcomes6.
To date, six BRS device companies (Abbott, Elixir Medical Corporation, ART, Biotronik, REVA Medical and Meril Life Sciences) have acquired CE mark approval for coronary artery disease devices. Most new-generation devices have a strut thickness of less than 100 μm. Eight BRS are in the pre-CE mark clinical trial phase, and another seven BRS are in preclinical assessment. Pre-regulatory clinical investigation of the BRS is especially prominent in Asian countries, where the BRS concept is philosophically and culturally attractive for the patients. The first CE-marked BRS was the Absorb™ scaffold (Abbott Vascular, Santa Clara, CA, USA) in January 2011 and the most recent approval by CE mark was for the MeRes100™ scaffold (Meril Life Sciences Pvt. Ltd., Vapi, India) in May 2019.
Advantages of magnesium and the Magmaris scaffold
The Magmaris® (Biotronik, Berlin, Germany) sirolimus-eluting scaffold is made of a magnesium backbone and polymer coating eluting sirolimus with a strut thickness and width of 150 μm, with 95% magnesium resorption at 12 months7,8,9,10. Magnesium has advantages over PLLA as a biodegradable material. Compared to poly-L-lactide or poly-D,L-lactide, magnesium has superior mechanical properties with a higher tensile strength and greater % elongation at break11. The magnesium alloy used in the Magmaris offers higher deformation resistance and lighter weight as compared to pure magnesium12. Several elements, such as aluminium, calcium, manganese, rare earth elements, yttrium, zinc, and zirconium, can be combined with magnesium to modify the mechanical properties (e.g., radial strength, hardness, etc.) and biophysical characteristics (e.g., degradation speed) of the magnesium-based alloy12. In addition, magnesium itself is known to have a low thrombogenicity12, presumably due to the fact that magnesium is negatively charged and may repel negatively charged platelets. In a preclinical study using an arteriovenous shunt (carotid-jugular) in a porcine model13, the Magmaris scaffold had significantly less platelet and inflammatory cell adherence and less thrombus deposition (5% vs 16.1%, p=0.02) than the Absorb. Furthermore, the Magmaris scaffold produced significantly less inflammatory cell adhesion when compared with the Orsiro stent (Biotronik). In a similar porcine model, Magmaris had less thrombogenicity and inflammatory cell deposition compared to a 316L stainless steel stent with the same geometry and design10,13.
BIOSOLVE-II, BIOSOLVE-IV and MAGSTEMI trials
The Magmaris device received CE mark approval in May 2016. In a serial angiographic follow-up of the BIOSOLVE-II trial with 123 patients enrolled, six- and 12-month angiographic in-scaffold late loss was 0.37±0.25 mm and 0.39±0.27 mm, respectively14,15. In a pooled population of the BIOSOLVE-II and BIOSOLVE-III trials (184 patients), the two-year target lesion failure rate was 5.9% without any definite/probable scaffold thrombosis (ScT) at the early or late/very late phases16.
In the current issue of EuroIntervention, three-year outcomes of the BIOSOLVE-II trial and one-year results of the initial 400 patients of the BIOSOLVE-IV trial are reported17,18. At the three-year follow-up of the BIOSOLVE-II trial including relatively simple lesions, a target lesion failure (TLF) rate of 6.8% (n=8) was observed with no probable or definite ScT. From one to three years, a mild increase of in-stent and in-segment late lumen loss (0.11±0.28 mm and 0.13±0.30 mm) was documented, resulting in a three-year in-device and in-segment minimal lumen diameter of 1.90±0.43 mm and 1.87±0.43 mm, respectively. Of note, the magnesium scaffold or its footprint (amorphous calcium phosphate) was no longer discernible by optical coherence tomography (OCT) or intravascular ultrasound (IVUS). Serial intravascular imaging by IVUS and OCT demonstrated stable lumen dimensions beyond 12 months (Figure 1).
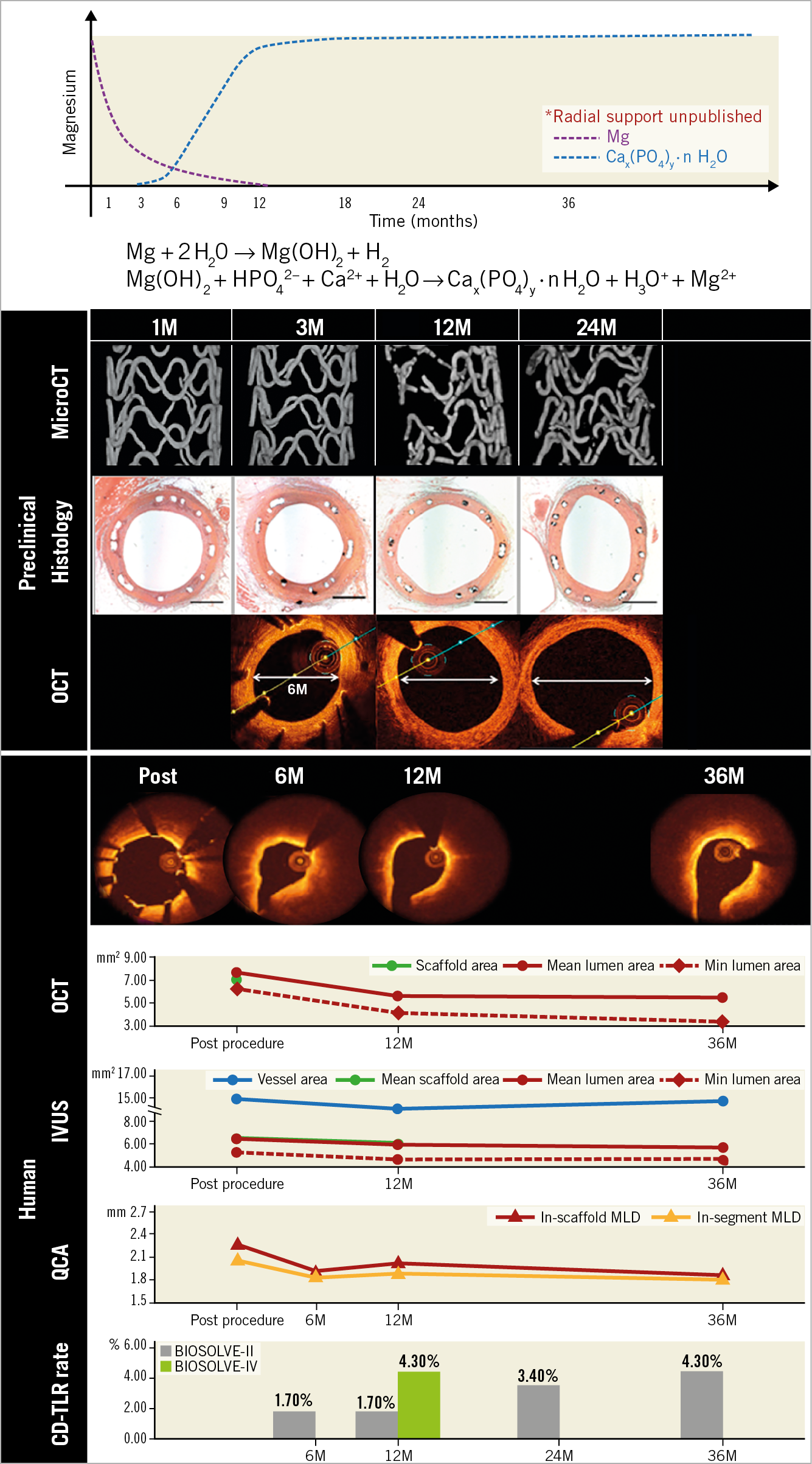
Figure 1. Preclinical and clinical results of the Magmaris scaffold. Upper panel shows the biodegradation profile of the Magmaris scaffold (modified from Sotomi et al11). The Magmaris is bioresorbed in approximately 12 months, by conversion to hydrated magnesium oxide, then to amorphous calcium phosphate. In a preclinical study (mid panel), micro-CT images demonstrate the dismantling of a scaffold or the degraded product (calcium phosphate) at around 12 months, which corresponds to the late enlargement of the lumen (modified from Waksman et al7 and Joner et al9). OCT images at 6, 12 and 24 months in the preclinical study are shown. In the BIOSOLVE-II study (lower panel), the struts start to become indiscernible on OCT at six months, and the multimodality imaging shows the stable lumen area from 12 months to 36 months (reproduced from Haude et al17). QCA data were based on non-paired analysis (Supplementary Table 3 in Haude et al17), whereas OCT and IVUS data were from paired analysis (Table 2 in Haude et al17).
BIOSOLVE-IV is an international single-arm registry which will include 2,054 patients. The one-year results of the first 400 patients are reported as a pre-specified interim analysis18. At one year, the TLF rate was 4.3%, driven by clinically indicated target lesion revascularisation (Figure 1). One subacute, definite ScT was observed after implantation of the Magmaris in a setting of post non-ST-elevation myocardial infarction; this early thrombosis was presumably related to the early cessation of antiplatelet therapy for planned coronary artery bypass grafting (CABG).
Although the clinical outcomes from these two registries were observed in simple lesions, it is remarkable that only one thrombotic event was documented throughout these clinical investigations (1/523, 0.2% - if these two studies are simply pooled).
Recently, Sabaté et al investigated vasomotion in the MAGSTEMI randomised trial comparing the Magmaris (N=73) and the Orsiro metallic stent (N=76) in a setting of ST-elevation myocardial infarction treated with primary PCI; in-device vasomotion at one year was significantly more intense in the Magmaris arm than in the Orsiro group, testing both vasodilation in response to nitroglycerine and vasoconstriction in response to acetylcholine19. However, in-device late lumen loss was significantly lower in the Orsiro group (0.61±0.55 mm vs 0.06±0.21 mm; p<0.001). The device-oriented composite endpoint was higher in the Magmaris arm, driven by an increase in ischaemia-driven target lesion revascularisation (16.2% vs 5.2%, p=0.030). The definite thrombosis rate was similar between the groups (Magmaris: 1.4%, Orsiro: 2.6%; p=1.0).
Potential mechanisms of early, late/very late thrombosis of the first-generation polymeric Absorb scaffold: are they not operational in the Magmaris scaffold?
The first-generation everolimus-eluting polymeric scaffold, the Absorb scaffold, raised a safety concern due to the increased ScT rates compared to metallic everolimus-eluting stents. In a patient-level meta-analysis including all randomised clinical trials with patients with chronic coronary syndromes (CCS)20 (ABSORB II, ABSORB III, ABSORB China and ABSORB Japan)21 including 3,389 patients (BRS 2,164 vs CoCr-EES 1,225)22, the TLF and ScT rates with the Absorb were significantly higher at three years compared to the CoCr EES (14.9% vs 11.6%, p=0.03 and 2.5% vs 0.8%, p=0.002, respectively). After three years, the increased risk of the Absorb compared to the XIENCE (Abbott Vascular) seemed to have subsided after the complete bioresorption of the scaffold. From three to five years, the event rates appeared to be non-significantly different between the two devices, and numerically fewer ScT occurred with the Absorb after three years.
The mechanism of early ScT is mainly related to the thrombogenicity of the polymeric scaffold struts protruding into the lumen at the time of the dismantling of the scaffold. The process is facilitated by the relatively thick strut thickness (150 µm) and widths that impede the embedment of the struts into the vessel wall23. The late/very late ScT is related to the partial absence of tissue encapsulation of the scaffold struts into the vessel wall between two and three years, which could lead to the intraluminal protrusion of the bioresorbable materials which, at two to three years, as demonstrated in histopathology, is replaced by provisional matrices of proteoglycan, etc., that are eminently thrombogenic24,25,26. The initial strut embedment and late encapsulation may, to a certain degree, be facilitated by the implantation technique (predilatation, sizing and post-dilatation)27.
This “vulnerable” period of intraluminal dismantling (two to three years for the Absorb scaffold) is related to the actual duration of bioresorption of the scaffold. It occurs in the last phase of bioresorption during which the scaffold loses its mechanical support and integrity and becomes dismantled as part of the programmed bioresorption process. To prevent the intraluminal dismantling, it is of paramount importance to achieve complete tissue “encapsulation” of the struts before this vulnerable period.
For a magnesium scaffold, this potential vulnerable period of dismantling is around six to 12 months after implantation, a period during which the patients are still protected from thrombotic events by dual antiplatelet therapy (Figure 1). Thrombotic events have not been observed in the clinical studies so far conducted with the Magmaris. In contrast, the shorter time period of bioresorption could lead to an early loss of mechanical support, potentially resulting in scaffold “late recoil” and restenosis28.
Future perspectives
The results of the BIOSOLVE-II, BIOSOLVE-IV and MAGSTEMI studies highlight the fact that there is no “class effect” in BRS; the metallic magnesium BRS does not raise any concerns of increased ScT. The magnesium backbone certainly has advantages in preventing ScT with its antithrombotic material properties, relatively higher mechanical properties and its shorter bioresorption time. The vulnerable time of bioresorption is most likely covered with dual antiplatelet therapy. However, as demonstrated in the MAGSTEMI trial, there are signs of increased revascularisation rates mainly due to a high late loss, which may be related to the early loss of mechanical properties28.
The remaining clinical questions are: i) what is the performance of the device in complex lesions; and ii) could the relatively high restenosis rate observed with the Magmaris justify the use of this scaffold in preference to the current drug-eluting stent with differential long-term benefit related to the Magmaris? The other technical question is whether the device could (or should) be improved in terms of the thickness and deliverability. We are one step closer to our dream, but still more clinical evidence (randomised controlled trials) is needed to merit the return of the BRS after its historical setback.
Conflict of interest statement
P.W. Serruys reports personal fees from Sino Medical Sciences Technology, Philips/ Volcano and Xeltis, outside the submitted work. The other author has no conflicts of interest to declare.

