Abstract
Aims: There is little in vivo data in regards to the impact of adventitial neovascularisation on vascular remodelling and plaque composition. Using a porcine model of coronary atherosclerosis, we aimed to determine the impact of adventitial neovascularisation on plaque composition and vascular remodelling evaluated by IVUS.
Methods and results: Coronary atherosclerosis was induced by adventitial delivery of lipids and a high cholesterol diet. At termination all vessels were analysed using IVUS to determine the degree of remodelling of each individual segment containing atherosclerotic lesions. Then, each segment was correlated with its correspondent histological frame for plaque composition and neovessel density. A total of 57 atherosclerotic lesions at different stages of development were analysed. The total neovessel count (TNC) correlated to the degree of plaque burden (15.6±7.2 TNC in <40% stenosis versus 35.7±14.0 TNC in >60% stenosis, p<0.01) and to the amount of intra-plaque collagen (32.4±14.1%, lower TNC tertile versus 47.5±8.9% upper TNC tertile, p< 0.01). The amount of intra-plaque SMC content inversely correlated with the TNC (49.7±18.9% versus 36.4±14.4%, lower versus upper tertiles, p<0.05). Plaques with the highest TNC showed higher remodelling indexes by IVUS (0.89±0.32 in lower TNC tertile versus 1.36±0.73 in upper TNC tertile, p<0.05) and higher macrophage cell content (161.42±157.6 in lower TNC tertile versus 340.6±127.2 in upper TNC tertile, p<0.05) compared to non-remodelled segments.
Conclusions: Adventitial neovascularisation is more prominent in positively remodelled segments and appears to be associated to SMC loss, increase collagen deposition and localised macrophage infiltration.
Introduction
Adventitial vasa vasorum are functional end arteries naturally occurring in the bifurcation areas of the epicardial arteries or the main lumen of the aorta1,2. Although already present at birth, the extent and distribution of this vascular system proportionally increases in density as the vessel wall grows and is believed to play a central role on vessel wall nutrition and cell trafficking3,4. Several pathology-based studies have demonstrated a strong correlation between vasa vasorum-derived neovascularisation and atherosclerotic plaque formation and destabilisation5,6. However, although the pathologic observation of plaque angiogenesis in atherosclerosis is well documented in the literature, the biological impact of such angiogenesis on plaque remodelling and composition is still unclear.
The potential detection of plaque neovascularisation presents a new window of opportunity in the field of cardiovascular imaging7-9. However, it is still unclear if vascular segments displaying enhanced neovascularisation are associated to the presence of more complex atherosclerotic lesions. We have previously published our preliminary experience in regards to the development of atherosclerotic lesions by the injection of cholesterol esters and complex lipoproteins into the adventitia of porcine coronary arteries10,11. In the present study, following lesion induction in this model, we evaluated all vascular segments using intravascular ultrasound (IVUS) and compared the resulting remodelling indexes with plaque composition and total neovessel count.
Methods
Experimental animal model
A total of five purpose-bred pigs were used in this experiment. The methodology and procedural description of the model utilised have been previously published10,11. The study protocol was approved by the local institutional animal care and use committee. All animals received humane care in compliance with the Animal Welfare Act and the “Principles of Laboratory Animal Care” formulated by the Institute of Laboratory Animal Resources (National Research Council, NIH Publication No. 85-23, revised 1996). Two weeks after the initial balloon injury, intra-mural delivery of a liposome-based formulation of complex lipids was performed in two major epicardial coronary arteries in three different but adjacent locations (30 segments, 10 arteries) using a microsyringe infusion catheter (Mercator MedSystems, San Leandro, CA, USA). In a modification of the previously described method, human oxidised LDL isoform-5 was added to the cholesterol ester injectate. After coronary angiography, the injection site was selected following intravascular ultrasound (IVUS) analysis. The catheter was placed over a coronary wire and the intra-mural delivery of the atherogenic mixture was performed over a 20 mm segment. Quantitative coronary angiography (QCA) was performed at baseline and immediately prior to termination of the study using Siemens Quantmas software integrated into digital angiography equipment (Siemens Axiom Artis; Siemens AG, Munich, Germany). Following the procedure, all pigs were returned to recovery rooms and maintained on a high cholesterol diet (2% cholesterol, 20% lard and 1.5% sodium cholate) for approximately 12 weeks after injection.
LDL-charged fractionation
LDL used for the injectate was isolated from homozygotic familial hypercholesterolemic (FH) and normolipidemic subjects and separated according to charge using a LCC-500 programmer controlling two P-500 pumps on an UnoQ12 column, an anion exchange column (BioRad; Hercules, CA, USA) pre-equilibrated with buffer A (0.02 M Tris-HCl, pH 8.0, 0.5 mM EDTA) at 4 °C. LDL-protein in buffer A was loaded onto the UnoQ12 column and eluted with a multi-step gradient of buffer B (1 M NaCl in buffer A): 0%, 10 min; 0-15%, 10 min; 15%-20%, 30 min; isocratic 20%, 10 min; 20-40%, 25 min; 40-100%, 10 min; 100%, 15 min; 100-0%, 5 min and 0%, 25 min. LDL fractions were pooled according to NaCl concentration into five subfractions, L1 through L5 (0.08-0.17 M, 0.17-0.18 M, 0.18-0.20 M, 0.20 M and 0.20-0.38 M, respectively). The isolates were concentrated, sterilised, and stored at 4 °C.
Intravascular ultrasound imaging
All IVUS images were acquired using a 20MHz Volcano Eagle Eye™ IVUS catheter (Volcano Therapeutics Inc., Rancho Cordova, CA, USA). Heparin and intra-coronary nitroglycerine were administered before imaging. Using angiography and post-intervention record for guidance, the IVUS catheter was inserted distal to the lesion and manually pulled back to assess the severity and length of the lesion. The IVUS catheter was then placed distal to a side branch (distal fiduciary landmark site) and automatic pullback was performed at a rate of 1 mm/sec gated to a continuous EKG. The location of the IVUS catheter was determined using continuous fluoroscopy and by recording anatomical landmarks seen during IVUS imaging, then every IVUS frame was individually matched with its correspondent histological slide. Two pullbacks per artery were performed and the best play loop was chosen based on resolution and quality. A complete morphometric analysis was performed using standard IVUS definitions12. To determine vascular remodelling the reference frame was calculated as the average between the most normal-looking cross-sections within 15 mm both proximally distally of the maximal stenosis, without interposition of a side branch13.
Histology protocol
All animals were euthanised at 12 weeks, the heart retrieved and the coronary circulation anterogradely perfused with one litre of heparinised normal saline at 100 mmHg. Individual coronary arteries were excised and the matching fiduciary distal landmark identified. Sequential 2 mm sections were obtained starting at the same anatomical position in which IVUS pullback was started in the segment. A total of 10 corresponding histological blocks were generated and placed in individual histology cassettes labelling their orientation and landmarks. The tissue was snap-frozen on liquid nitrogen and sent for processing. Serial 5-µm sections were cut from each histological block and stained with haematoxylin and eosin, Oil-red-O, Masson’s trichrome/elastin stain and Movat’s pentachrome. Histological classification of the lesions was performed using standard definitions14.
Immunohistochemistry protocol
Immunohistochemical staining was performed using a myeloperoxidase-labelled streptavidin-biotin method from Neomarkers (Neomarkers Inc., Fremont, CA, USA) to detect inflammatory cells. Sections were also immunolabelled with an anti-factor VIII antibody followed by a standard avidin-biotin peroxidase complex assay Vector ABC Elite Kit (Vector Laboratories Inc., Burlingame, CA, USA) to stain neovessels, considered as an acceptable technique for neovessels identification15. Slides were developed with diaminobenzidin (DAB, Zymed Laboratories, San Francisco, CA, USA) and counter stained with 10% haematoxylin. Following staining, 24-bit, full-colour digital images were using an Olympus U-CMAD3 camera (Olympus America, Inc., Melville, NY, USA) attached to an Olympus BX41 microscope (Olympus America, Inc., Melville, NY, USA) at a resolution of 1024x768 pixels. Morphometric analysis was performed using Image-Pro plus 4.5® (Media Cybernetics, Silver Spring, MD, USA). The characterisation of morphological components was performed by previous standards16. The individual total plaque area and individual plaque components were recorded for each slide and the sum of all of the components matched to the total plaque area.
Neovessel quantification
Plaque neovascularisation was assessed by counting the number of individual neovessels present in the adventitia in each histological section (TNC: total neovessel count). Each slide containing the arterial segment was divided into quadrants in order to include the entire histological slide. The number of vascular structures positively stained with an anti-factor VIII antibody within the adventitial layer were identified as neovessels and quantified as absolute number per plaque using Image-Pro plus 4.5.
Statistical analysis
Histological morphometry measurements were stratified into tertiles based on the degree of adventitial neovascularisation. Normal vascular segments that were not injected were used as the control group. The data are presented as mean±SD for parametric data and percentages for categorical variables. Comparisons between continuous variables were performed using one way ANOVA as data was normally distributed, as per the Kolmogorov Smirnov test. Comparisons between categorical variables were performed using the chi-square test. Statistical analysis was performed using SigmaStat 3.11 software, 2004 (Systat Inc., San Jose, CA, USA) and Matlab R2008a (The Mathworks, Inc., Natick, MA, USA). A P-value of 0.05 or less was considered statistically significant.
Results
A total of 30 coronary segments were injected with a lipidic mixture (15 in right coronary arteries, 15 in left anterior descending arteries) resulting in the development of 57 atherosclerotic lesions at different stages of development. The control group (non-injected segments) included a total of 10 crosssections randomly selected from the coronary vessels including segments from the left anterior descending, right coronary and circumflex arteries. The mean total cholesterol level achieved at follow-up by dietary intervention was consistently between 300 and 400 mg/dl among all animals. All arterial segments were angiographically analysed at the time of termination. There was no evidence of ulceration, calcification or thrombus formation in any of the lesions by angiography. In the injected segments, QCA showed a percentage diameter stenosis of 13.7±5% and a lesion length of 16.6±6.9 mm. IVUS analysis showed a mean plaque area of 6.70±1.50 mm2 and a plaque burden of 41.10±6.99%. The mean arterial remodelling index of the analysed segments was 1.06±0.56 and IVUS criteria for positive remodelling were found in half of the frames (50.9% of injected segments). There was no evidence of positive remodelling in any of the non-injected control segments.
IVUS-histology correlation analysis
A total of 57 coronary atherosclerotic lesions at different stages of development were identified by IVUS and classified by histology. The mean plaque area found was 1.90±0.93 mm2, with a mean plaque burden of 50.66±14.78%. The largest plaque area and plaque burden were 5.01 mm2 and 78.36% respectively. The mean EEL area was 3.73±1.36 mm2 and the mean lumen area was 1.82±0.85 mm2. Fatty streaks were found in 8.7% of the cases, pathological intimal thickening in 54.5% and fully developed fibro-atheromas in 36.8% of the cases. Among these, the mean area occupied by collagen was 39.59±12.8% and by lipids 17.11±8.99%. The mean neovessel count per plaque in all the lesions was 24.79±13.09 and was significantly higher compared to the control segments (2.5±2.8 neovessels count per plaque, p < 0.001). The mean number of macrophages found in all lesions by immunohistochemistry staining was 244.86±146.84 cells/plaque. The degree of macrophage infiltration was maximal in lesions with the highest plaque burden when divided in ascending tertiles by the percentage area of stenosis (123.42±146.16 macrophages in the tertile with the lowest plaque burden, 267.14±102.03 in the middle tertile, and 331.62±116.67 in highest tertile, p=0.01, Figure 3A).
Neovascularisation and plaque composition
The number of neovessels per plaque segment increased proportionally to the degree of area of stenosis (15.55±7.18 mean neovessel count for plaques with <40% stenosis, 35.70±14.03 mean neovessel count for plaques with ≥60% stenosis, p=0.001, Table 1).
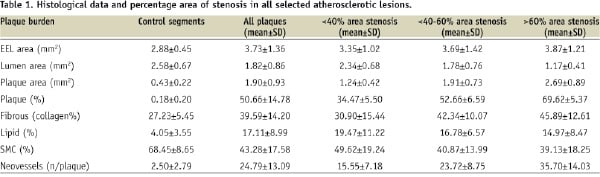
Plaques in the highest tertile of adventitial neovascularisation displayed the biggest plaque burden (65.2±9.4%) compared to the middle (49.7±14.9%) and the lowest neovascularisation tertiles (41.6±11.7%, p <0.001, Figure 1A). Even when compared to the segments with less plaque burden (<40%), the control group demonstrated significantly less total neovessel count (2.5±2.8 in control segments vs. 15.5±7.2 in low plaque burden segments, p <0.001). Similarly, the deposition of intra-plaque collagen was higher as the density of adventitial neovascularisation increased (47.5±8.9% for the upper tertile, 39.3±15.1% for the middle tertile and 32.4±14.1% for the lower tertile, p=0.005, Figure 1B). By the contrary, lower intra-plaque smooth muscle cell content was seen in plaques containing a higher degree of adventitial neovascularisation (49.7±18.9% smooth muscle cell area in the lower tertile versus 36.4±14.4% smooth muscle cell area in the upper tertile, p=0.026, Figure 1C). The percentage area occupied by the lipids was similar within the three analysed tertiles, (p=0.85, Figure 1D).
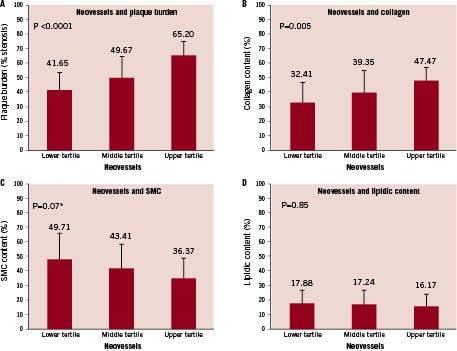
Figure 1. Effect of adventitial neovascularisation on plaque composition. Panel A: Plaque burden and neovessels. Panel B: Collagen content and neovessels. Panel C: Smooth muscle cells and neovessels. Panel D: Lipid pool and neovessels.
Similarly, macrophage infiltration correlated with the degree of neovessel density (161.42±157.6 macrophages per plaque for the lower tertile, 218.8±102 in the middle tertile and 340.6±127.2 in the upper tertile, p=0.044, Figure 3B). Accordingly, the number of macrophages present in the control segments was minimal (0.4±0.5 macrophages per plaque) compared to the segments that contained in type of atherosclerotic lesion.
Neovascularisation and vascular remodeling
Positive remodelling evaluated by IVUS was most commonly seen among the plaques containing the highest amount of adventitial neovascularisation (remodelling index = 0.89±0.32 in the lower tertile, 0.92±0.47 in the middle tertile and 1.36±0.73 in the upper tertile, p=0.02, Figure 2).
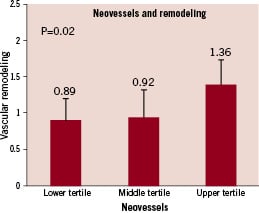
Figure 2. Association of adventitial neovascularisation with vascular remodelling.
The histological sections from the control segments did not show any signs of positive vascular remodelling, mirroring the findings found during in vivo IVUS imaging. Positive vascular remodelling evaluated by IVUS did not correlate with the presence of any other plaque components. However, the mean number of macrophages trended to be higher in positively remodelled segments but this difference did not show statistical significance (269.84±156.12 macrophages per plaque in positive remodelled segments vs. 208.77±132.525 macrophages in non-remodelled segments, Figure 3C).
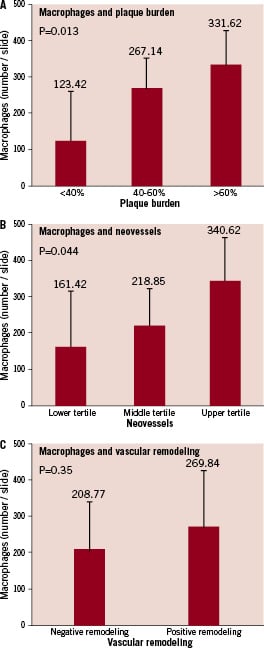
Figure 3. Macrophage infiltration, vascular remodelling and neovascularisation. Panel A shows the relationship of macrophage infiltration on plaque burden. Panel B shows the relationship of plaque neovascularisation on macrophage infiltration. Panel C shows the relationship of macrophage infiltration and vascular remodelling.
Discussion
Pathological arterial wall neovascularisation mainly derived from adventitial vasa vasorum appears to participate in the progression and destabilisation of atherosclerotic vascular disease5,6. Although the biological mechanisms associated to this pathological finding are still unclear, it appears that the uncontrolled formation of neovessels promotes the formation of rupture-prone plaques17. Although the pathologic observation of this finding is well documented in the literature, it is still unclear if intra-plaque neovascularisation has a direct impact on vascular remodelling and atherosclerotic plaque composition or if this finding is simply a bystander innocent resulting from a continuous biological process.
In the present study, we aimed to determine the impact of adventitial neovascularisation on vascular remodelling and plaque composition among lesions found by IVUS at different stages of development. For this purpose, we used an animal model of coronary atherosclerosis previously described by our group10,11. By using this approach, we took advantage of the fact that the lesions are followed prospectively, are developed within similar vascular segments and provide normal control segments for comparison. In addition, we believe this study design is a step forward from the previously published work in swine in which a non-diseased swine with minimal plaque formation was used18. In this study, we chose LDL5 as the source of the injectate as this molecule has shown to up regulate adhesion molecules and CXC chemokines inducing apoptosis in human umbilical venous endothelial cells (CYY, CMB)19.
Our in vivo findings support previously published autopsy human studies showing that adventitial neovascularisation contributes to the process of plaque formation. In our study, normal coronary segments harvested from adjacent sites containing atherosclerotic lesions contained minimal amounts of adventitial neovascularisation. The angiogenic process driving this phenomenon appears to be triggered by intra mural hypoxia that limits luminal oxygen diffusion and augments the formation of immature intra-plaque vascular channels from the adventitial layer. Vascular Endothelial Growth Factor (VEGF) and Platelet-Derived Endothelial Cell Growth Factor (PD-ECGF), expressed by smooth muscle cells and foamy macrophages located in the intima, may play a role in the formation of the new vascular channels20 that are thought to grow in an “outside-in” fashion21. The recruitment and formation of these vascular structures promote the migration of different cells, such as fibroblasts, pericytes and inflammatory cells22. This model differs from previous studies in which the investigation of adventitia vasa vasorum employed small animal models usually at non-coronary vessels23 and from other large animal models in which the lesions obtained by diet or angioplasty lacked higher similarity with human atherosclerosis18,24,25. Therefore we consider that our model provides a unique opportunity to study the mechanisms of plaque angiogenesis in vivo as plaques can be induced in any specific segment of the arteries and the progression followed using different endovascular imaging tools.
Adventitial neovascularisation appears to modulate extracellular matrix composition during atherosclerosis plaque development. Our findings are consistent with Jeziorska et al, showing the effect of neovascularisation on plaque composition and the association with areas prone to rupture in human plaques26. In our study, the density of plaque neovascularisation was associated with a higher degree of collagen deposition and lower smooth muscle cell content in the plaques, a finding consistent with previous reports that correlate the progressive loss of smooth muscle cells as the atherosclerotic lesions progresses toward a more complex state27. These findings might also reflect a more complex and advanced stages in plaque formation, as opposed to the earlier stages of atherosclerosis in which smooth muscle cells are present in a substantial amount in order to promote the synthesis of extracellular matrix components and collagen as a response to vascular injury28. Later in the course, as the plaques become more advanced, a decrease in the number of smooth muscle cells appears to be associated with extracellular matrix loss, enhanced inflammation necrotic core formation and fibrous cap thinning29. The mechanisms by which these cells are lost in advance stages of the plaque are not well known, but might include apoptotic cell death30. In contrast, some authors have shown that neovessels and smooth muscle cell density increase proportionally as atherosclerosis develops31. This biological process does not conflict with our data, as this “cellular switch” was clearly demonstrated in lesions at different stages or development and may represent a temporal change during the process of plaque development. In any case, the role of smooth muscle cell transformation in plaque instability and its relation to neovascularisation and plaque remodelling needs to be studied in further detail.
Another important aspect of this study was the relationship between the plaque neovascularisation and positive vascular remodelling. The direct biological effect of adventitial neovascularisation on vascular remodelling has not been evaluated in a large animal model of coronary lesions as previous large animal models featured only very early disease or include arterial segments undergoing angioplasty and presenting constrictive remodeling24. In our study, we were able to analyse in vivo positively remodelled segments and select those segments to correlate with histological analysis (Figure 4).
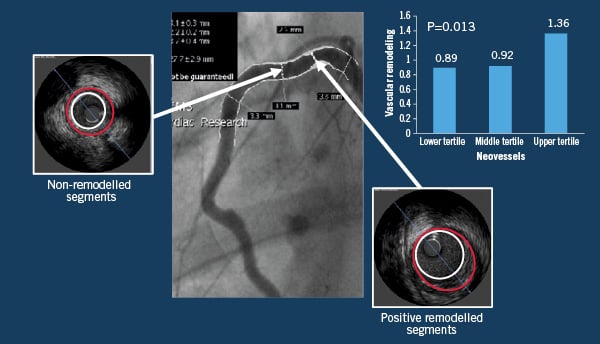
Figure 4. Association of in vivo vascular remodelling with adventitial neovascularisation. Representation of both remodelled and non-remodelled vascular segments that were analysed and compared in terms of plaque composition and neovessels density.
As seen in the clinical setting, we found higher index of vascular remodelling in plaques with higher levels of atherosclerotic burden32. We also observed a linear relationship between positive vascular remodelling, degree of neovascularisation and macrophage infiltration, a finding previously reported in autopsy specimens33. Interestingly, vascular remodelling was not associated to collagen presence or smooth muscle cell loss. A relevant biological aspect is the relationship between macrophage infiltration and plaque neovascularisation. In small animal models, it has been reported that cell trafficking plays a role in the development of these vessels by producing angiogenic cytokines and pro-angiogenic factors34. Despite the fact that it is not entirely clear yet whether plaque inflammation precedes angiogenesis, or if plaque angiogenesis is driven by macrophage infiltration per se, the combination of inflammatory cell infiltration and increased plaque neovascularisation has been associated with characteristics of plaque instability such as intraplaque haemorrhage17.
The limitations of this study are based on its experimental nature and the existing biological differences between human and porcine atherosclerosis. Additionally, the short development time of the atherosclerotic lesions and the selection of only one time point provides only a glance of the entire biological process. Similarly, the lack of a regression or intervention arm supports our conclusions as an association more than a causal relationship. Finally, it is possible that the neovascularisation observed in the coronary plaques from our model was a consequence of a compensatory response, as part of the healing process, of the adventitia to the injury caused to the vessel wall with the lipid injections. Nonetheless we consider this very unlikely given the histopathological characteristics of the lesions and their similarities with human atherosclerosis, most likely reflecting a true pathological process occurring inside the endothelium.
In summary, by using a porcine model of coronary atherosclerosis, we demonstrated that positive vascular remodelling identified by IVUS is associated to enhanced neovascularisation and macrophage infiltration. These segments contain increased amount of collagen content and less smooth muscle cell density compared to non-remodelled segments. In addition, the presence of adventitial neovascularisation appears to affect the process of atherosclerosis plaque formation and vascular remodelling by modulating smooth muscle cell proliferation and extracellular matrix deposition. The molecular mechanisms involved in plaque angiogenesis need to be studied in further detail prospectively in order to understand their role in plaque vulnerability and more importantly plaque regression. Finally, despite the interventional nature of this animal model, it seems to provide a controlled environment for the testing of mechanistic hypothesis in this field and for the validation of imaging techniques developed to detect intra-plaque neovascularisation.

