
Abstract
Prospective identification of lipid-rich vulnerable plaque has remained an elusive goal. Intravascular photoacoustics, a hybrid optical and ultrasonic technology, was developed as a tool for lipid-rich plaque imaging. Here, we present the first in vivo images of lipid-rich coronary atherosclerosis acquired with this new technology in a large animal model, and relate them to independent catheter-based imaging and histology.
Introduction
Lipid-rich atherosclerotic plaques in the coronary circulation cause the majority of acute coronary syndromes (ACS) and cardiac deaths. Plaques that may trigger adverse events frequently have a lipid-rich necrotic core with a thin fibrous cap (thin-cap fibroatheroma [TCFA]), large plaque burden, and a small lumen1. Prospective detection of such “vulnerable” plaques, by imaging plaque morphology and composition, may enable pre-emptive interventions. Today’s clinically available technology cannot characterise all these features simultaneously2. Intravascular optical coherence tomography (IVOCT) has adequate resolution for imaging thin caps, but lipid detection is based on qualitative interpretation. Only intravascular ultrasound (IVUS) quantifies plaque burden. Near-infrared spectroscopy (NIRS) is validated for lipid-core plaque detection but lacks depth resolution.
Intravascular photoacoustic (IVPA) imaging has been proposed for comprehensive characterisation of coronary atherosclerotic plaques. In photoacoustic imaging, one sends a short laser pulse and receives the ultrasound signal generated by optical absorption (Figure 1). Thus, optical absorbers can be localised through the acoustic propagation delay. Plaque lipid has been a prominent IVPA target: by selecting appropriate laser wavelengths, a unique and specific lipid image can be generated. Multimodal IVPA/IVUS data provide a rich combination of structural and composition imaging. The resolution of IVPA is comparable with IVUS. In this study, improved catheter geometry3 and validation of an animal atherosclerotic model4 enabled the first IVPA images of coronary atherosclerosis in vivo, a crucial step in the clinical translation of this new modality.
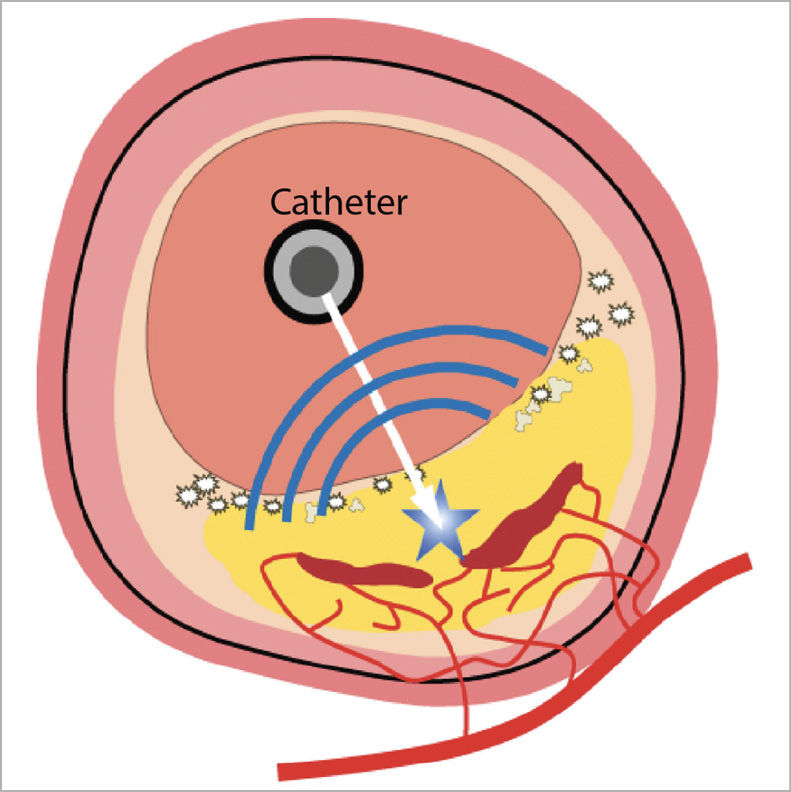
Figure 1. A hybrid optical/ultrasound catheter illuminates the vessel wall with nanosecond-pulsed infrared light. Chemically specific optical absorption generates ultrasound signals which can be detected, enabling imaging of tissue composition, including lipids
Methods and materials
The imaging system5 consisted of two pulsed lasers with wavelength λ ≈ 1,725 nm, ultrasound pulser, signal filtering and data acquisition hardware. A rotary joint (1,200 rpm) coupled the optical and electrical pulses to the IVPA/IVUS catheter (1 mm diameter), comprising an ultrasound transducer (40 MHz) and an optical fibre terminating with an angled mirror3 (Supplementary Figure 1). IVPA and IVUS signals were digitally filtered to suppress noise and artefacts.
Familial hypercholesterolaemia Bretoncelles Meishan (FBM) mini-swine developed atherosclerosis after nine months of a high-fat diet4. The present study, approved by the local animal welfare committee (DEC-EMC3125), was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The right coronary artery (RCA) and the left anterior descending coronary artery (LAD) were imaged using IVPA/IVUS, IVOCT and NIRS/IVUS. IVPA/IVUS pullback (1 mm/s) was performed under manual flushing of the artery with a saline solution from heavy water. All imaging was documented on angiography. After sacrifice, the coronary arteries were dissected from the epicardium, sectioned, and stained with Oil Red O.
Full methods are available in Supplementary Appendix 1.
Results
The LAD and RCA of an FBM mini pig were successfully imaged with IVPA/IVUS, NIRS/IVUS and IVOCT. NIRS/IVUS did not detect lipid-rich plaque in either artery; however, it revealed intimal thickening in the LAD and a near absence of abnormalities in the RCA. IVOCT confirmed these observations.
IVPA in the LAD distally (Figure 2) revealed a circumferential positive signal in the intimal layer (Figure 2A, Moving image 1). The IVPA signal exhibits the familiar “sawtooth” motion pattern of IVUS pullbacks (Figure 2B). This pattern confirms the tissue origins of IVPA signals in all data discussed here, as it is absent in artefactual signals. Circumferential intimal lipids, but no necrotic core, are present in a histological section from the imaged segment (Figure 2C). The intima in IVOCT is heterogeneous, but not strongly attenuating, suggestive of extracellular lipids or foamy macrophages. NIRS confirmed the absence of necrosis (Figure 2D).
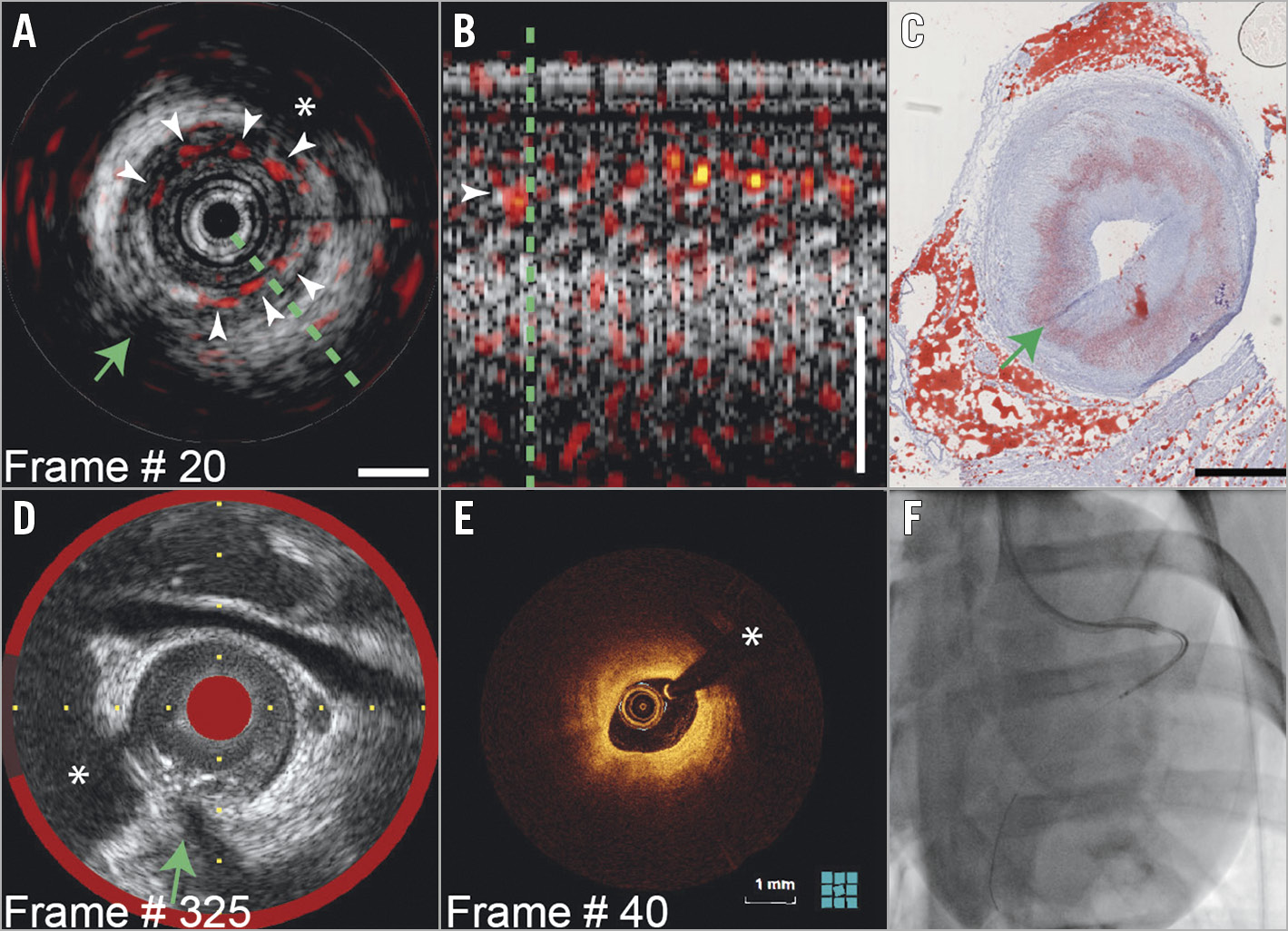
Figure 2. IVPA in the distal LAD (Moving image 1). A) IVPA images (red-yellow-white, dynamic range 20dB) on IVUS (greyscale, dynamic range 30dB, scalebar 1 mm). Intimal lipids generate IVPA signal (white arrowheads). B) Longitudinal section along the green line in A. C) Corresponding histology confirms presence of intimal lipids. D) Matched NIRS/IVUS shows no lipid core plaque. E) Matched IVOCT. F) Angiogram with radiopaque catheter. Green arrow: side branch ostium. *guidewire artefact.
In the proximal LAD (Figure 3), the IVPA signal indicates the focal presence of intimal (Figure 3B, white arrowheads) and peri-adventitial lipids (blue arrowheads). IVOCT (Figure 3A) shows signal-poor areas, consistent with extracellular lipid. Histology (Figure 3D, Figure 3E) demonstrates heterogeneously dispersed intimal lipid in this segment. Mild disease was found in the RCA (Figure 4), according to IVOCT (Figure 4A) and IVUS/NIRS (Figure 4C), with perivascular lipids evident on IVPA (Figure 4B, Supplementary Figure 2, Moving image 2).
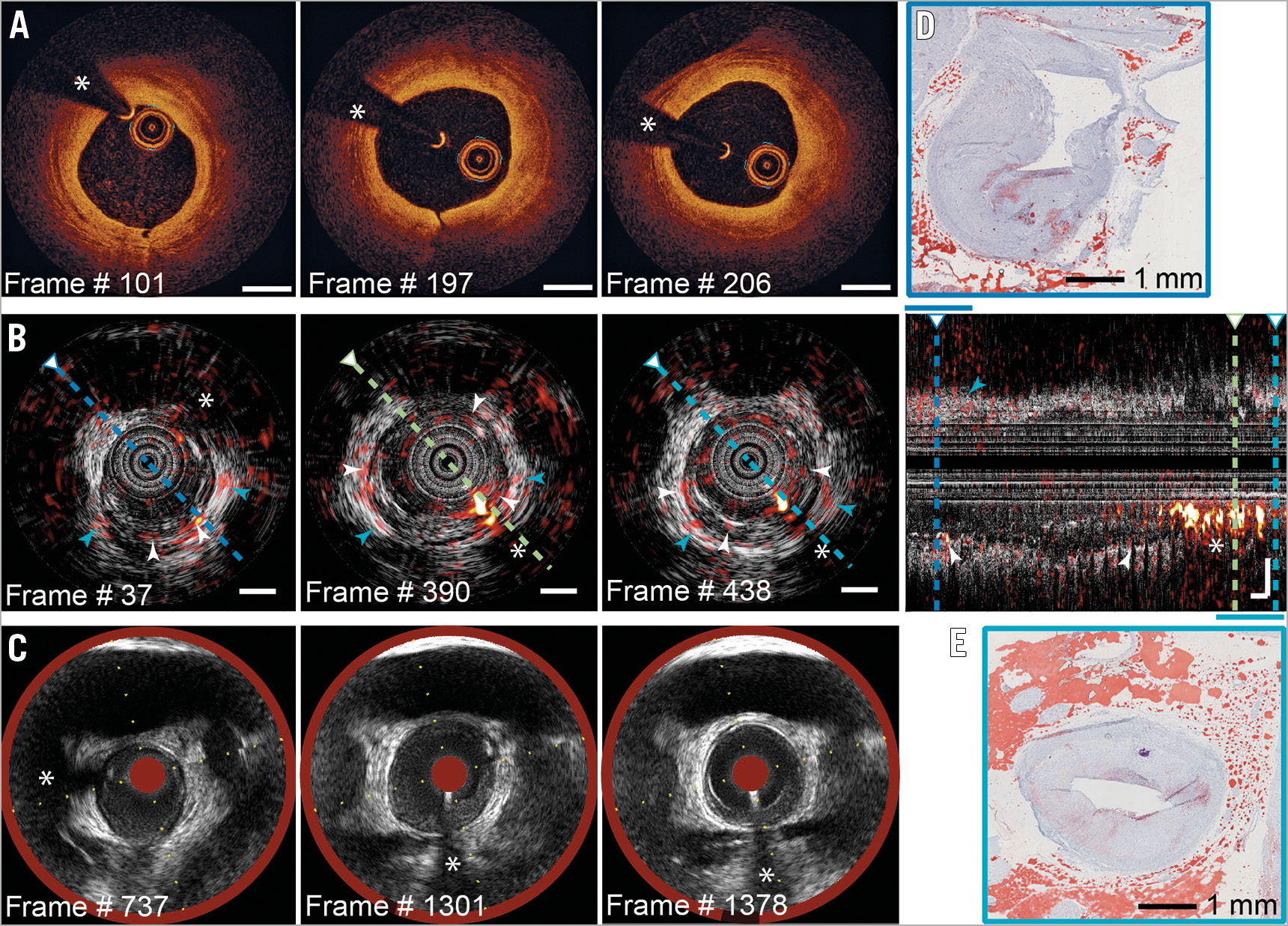
Figure 3. IVPA/IVUS in the proximal LAD. Selected matched frames: A) IVOCT; B) IVPA/IVUS, with longitudinal section; C) NIRS/IVUS. Histology in the distal (D) and proximal (E) segments confirms focal intimal lipids; cf. (B) (white arrowheads), and perivascular fat (blue arrowheads). Scalebars 1 mm; dynamic range as in Figure 2.
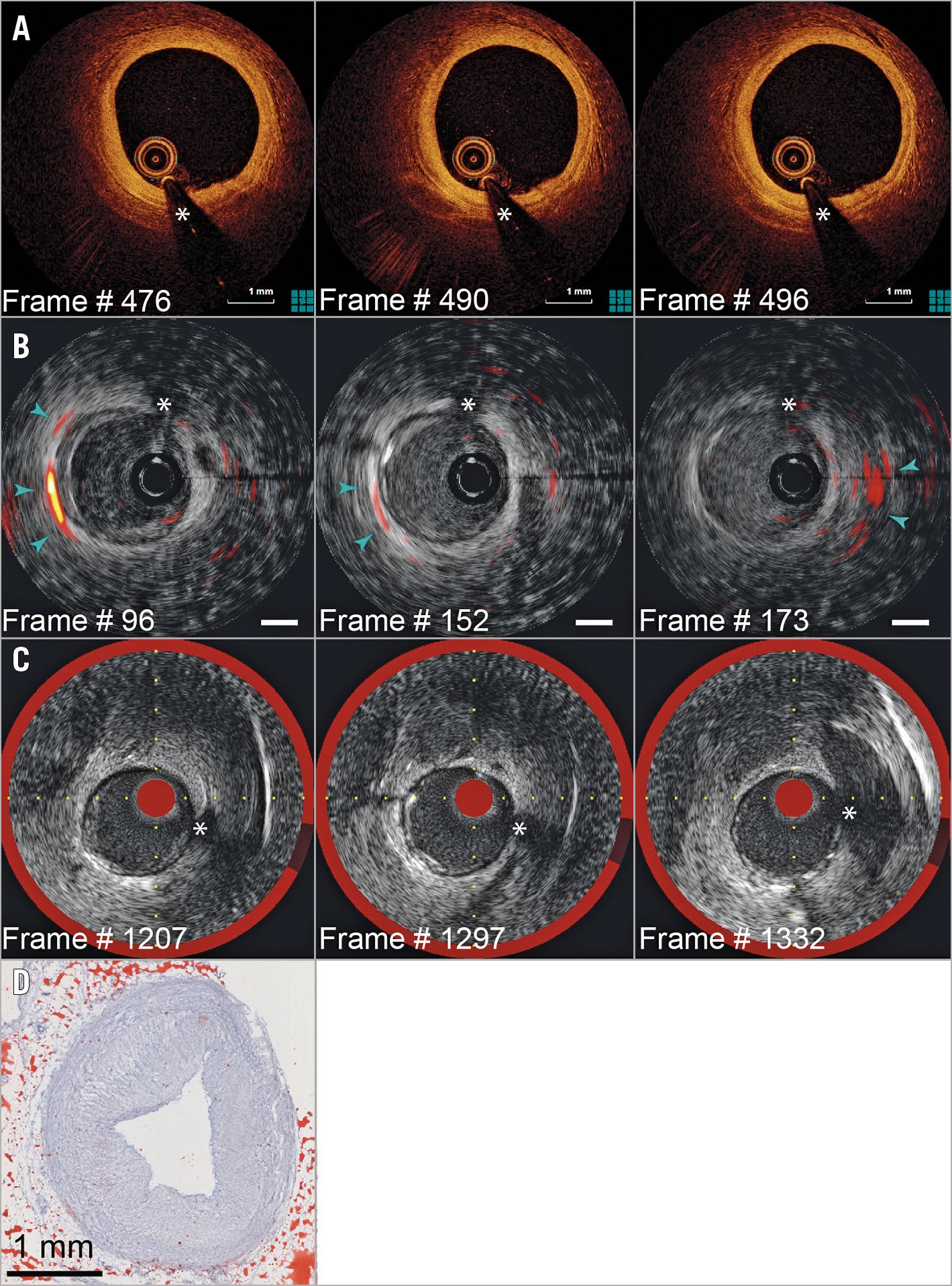
Figure 4. Pullback imaging in the RCA (Moving image 2, Supplementary Figure 2). Selected matched frames: A) IVOCT; B) IVPA/IVUS; the IVPA signal originates from peri-adventitial lipids (blue arrowheads). C) NIRS/IVUS. D) Histology confirms absence of plaque lipids. Scalebars 1 mm; dynamic range as in Figure 2.
Discussion and limitations
Lipids exhibit characteristic absorption bands allowing identification and localisation in tissue by photoacoustics. In this study, we demonstrate in vivo IVPA imaging of coronary lipid in an atherosclerotic swine. The imaging catheter and operation of the system are similar to IVUS, the difference being the application of saline flush. Further development of the technology (e.g., catheter sheath, noise-suppressing electronics and dual-frequency transducer) and evaluation in clinical trials are needed to establish the utility of IVPA and imaging criteria that may improve outcomes.
Imaging of lipid-rich plaque during PCI may improve our understanding of the risk of developing future ACS, and as such inform treatment strategy. Unlike NIRS, IVPA locates lipids relative to the lumen border, as shown in our data. Multi-wavelength IVPA distinguishes plaque lipid from perivascular fat. Enhanced ultrasonic sensitivity and noise immunity of the system will enable this distinction in future studies and also eliminate the need for flushing. In its present form, IVPA resolution is insufficient to measure cap thickness. One may assess plaque lipid “in contact with the lumen”, similar to IVUS-VH TCFA identification1.
Aspects of this preclinical study limit the scope of the conclusions which can be drawn from the results. We found extensive lipid-rich plaque, but no necrotic core according to histology or NIRS. Studies with IVPA on human autopsy specimens have demonstrated a strong signal from necrotic core plaque. Data were acquired in two vessels only; consequently, there are insufficient data to quantify the diagnostic accuracy of IVPA. Histology colocation was limited by the procedure of matching pullback location, angiography and frozen tissue blocks.
Conclusions
We have demonstrated IVPA imaging of coronary lipid-rich atherosclerotic plaque in vivo. In one artery, extensive plaque was found in which IVPA showed a positive signal for intimal lipids. Another artery exhibited mild intimal thickening with sparse or no lipid. Both findings were confirmed by IVOCT imaging and histology. A miniaturised, flexible catheter could be successfully deployed in the coronary circulation of a swine for pullback imaging, a crucial step in the translation of IVPA towards clinical trials.
|
Impact on daily practice This study highlights the potential for future clinical translation of a new catheter-based technology for imaging lipid-rich atherosclerotic plaque – intravascular photoacoustics. It detects lipids, but importantly it also locates them in depth in a structural IVUS image. In the future, such data may provide a prospective, lesion-specific risk assessment for adverse cardiac events. |
Acknowledgements
We thank Mathijs Stam, from the Department of Experimental Cardiology at Erasmus MC for preparing the histology.
Funding
Netherlands Organization for Scientific Research (NWO) 16131, 12706; European Research Council (310457).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Stationary scan of IVPA (red-yellow-white, dynamic range 20dB) and IVUS (greyscale, dynamic range 30dB, scalebar 1 mm) in the distal LAD; moving average over three cardiac cycles.
Moving image 2. Pullback recording of IVPA (red-yellow-white, dynamic range 20dB) and IVUS (greyscale, dynamic range 30dB, scalebar 1 mm) in the distal RCA displayed Supplementary Figure 2.

