
Abstract
Aims: This study aimed to establish the early healing and neointimal transformation profile of the new polymer-free BioFreedom stent through sequential optical coherence tomography (OCT) within the first nine months following stent implantation.
Methods and results: We randomly assigned 104 BFS recipients to one of five groups with angiography and OCT follow-up at 1, 2, 3, 4, or 5 months, together with another follow-up for all at nine months. The primary endpoint was the degree of OCT-detected strut coverage at nine months. From 1, 2, 3, 4, and 5 months, median neointimal strut coverage increased from 85.8, 87.0, 88.6, 96.8 to 97.1%, respectively, to 99.6% (IQR 98.2-99.9) at nine months. At nine months, median percent neointimal volume was 13.0% and angiographic late lumen loss was 0.21±0.30 mm. Major adverse cardiac events (MACE) were limited to one non-cardiac death, one non-ST-elevation myocardial infarction not related to BFS, and two target lesion revascularisations without stent thrombosis (MACE rate 4.0%).
Conclusions: Neointimal strut coverage of the BFS was rapid and the BFS was shown to be clinically safe and effective.
Abbreviations
BA9: biolimus A9
BFS: BioFreedom stent
DES: drug-eluting stents
LLL: late lumen loss
NIA: neointimal area
NIT: neointimal thickness
NIV: neointimal volume
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
Introduction
Drug-eluting stents (DES) offer a lower rate of restenosis1 than bare metal stents. However, late stent thrombosis due to delayed arterial healing, which may be related to durable polymers, has become a major concern2. To control this risk, extended antithrombotic regimens are used at the expense of increased rates of bleeding3. The need for more biocompatible stents, including those that modify or eliminate the polymer, has become increasingly important. Biolimus A9™ (BA9; Biosensors Europe SA, Morges, Switzerland) is a proprietary highly lipophilic semi-synthetic sirolimus derivative rapidly absorbed into tissue. When administered in equivalent doses, BA9 has a similar safety profile to sirolimus, while its substantially higher lipophilicity results in lower systemic drug concentrations compared with sirolimus-eluting stents (SES)4.
The new BioFreedom™ stent (BFS) (Biosensors Europe SA) uses a polymer-free design with a microstructured abluminal surface harbouring BA9, which is transferred to the vessel wall over a period of one month. In a preclinical study, the BFS caused less neointimal proliferation and inflammation at 180 days than an SES5. In a first-in-human measurement of 12-month in-stent late lumen loss (LLL), the BFS was non-inferior to a paclitaxel-eluting stent6. Finally, in the LEADERS FREE double-blind randomised trial, the BFS was significantly safer and more efficacious than a bare metal stent in patients at high bleeding risk7.
This study sought to establish the early healing and neointimal transformation profile of the new polymer-free BFS through sequential optical coherence tomography (OCT) within the first nine months following stent implantation.
Methods
PATIENT SELECTION AND STUDY DESIGN
In this prospective, single-centre, single-arm study, patients from 18-85 years of age with de novo coronary lesions requiring percutaneous coronary intervention (PCI) were consecutively enrolled at Queen Mary Hospital at the University of Hong Kong. Patients with ST-segment elevation myocardial infarction (STEMI) were excluded. The protocol was approved by the ethics committee of Queen Mary Hospital and all patients provided written informed consent. All patients underwent three OCT assessments: first, at baseline to ensure optimal stent implantation; second, at 1, 2, 3, 4, or 5 months in five randomly assigned groups for assessment of early stent coverage and healing; and third, at nine months for OCT and angiographic assessment of neointimal metrics (Figure 1). Clinical follow-up was scheduled at 1, 6, 9, and 12 months.
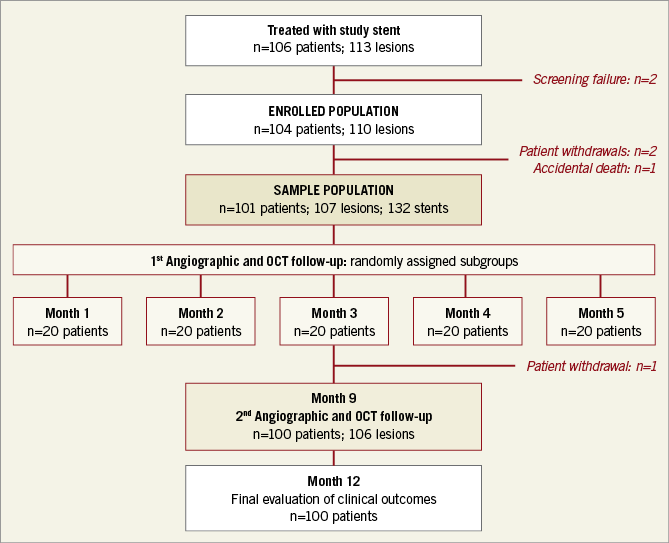
Figure 1. Flow chart of patients from screening to 12-month clinical follow-up.
STUDY DEVICE
The BFS has been described in detail previously4-6. In brief, it is a metallic, 316L stainless steel stent, treated to create a proprietary, selectively microstructured abluminal surface in which BA9, a highly lipophilic semi-synthetic derivative of sirolimus, is harboured without the use of a polymer or primer. For the study, the BFS was available in four diameters between 2.5 and 4.0 mm, and in five lengths between 11 and 33 mm, with the total dose of BA9 on the stent ranging from 178 to 521 µg.
PROCEDURES
PCI was carried out in the usual manner after administration of dual antiplatelet therapy (DAPT) (aspirin/clopidogrel) and unfractionated heparin. The BFS was deployed from “normal-to-normal” segment, with a diameter ratio of 1.1:1 vs. the reference. Overlapping stents were allowed for long lesions. Meticulous optimisation was adopted under full OCT guidance. Patients were discharged on a regimen of 80 mg aspirin and 75 mg clopidogrel daily for 12 months, followed by indefinite aspirin monotherapy.
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA)
Angiograms were obtained in two orthogonal, matching views before and after the index procedure and at follow-up. All angiograms were analysed offline by an independent core laboratory (Supplementary Appendix 1) blinded to the clinical information. For qualitative analysis, standardised definitions were applied8, including target vessel and lesion location, morphology and complexity, and estimates of coronary blood flow (Supplementary Appendix 2) using the QAngio XA 2D software, version 7.3 (Medis medical imaging systems, Leiden, the Netherlands).
OCT
All OCT images were collected with the C7-XR™ frequency-domain OCT system and Dragonfly™ imaging catheter (Abbott Vascular, Santa Clara, CA, USA) at a pullback speed of 20 mm/sec and a frame rate of 100 frames/sec using 14 to 16 mL of contrast. All OCT analyses were performed by a core laboratory (Supplementary Appendix 1) blinded to clinical information including the imaging time point using the Offline Review Workstation (Abbott Vascular). A pre-specified, six-category, early-coverage classification was evaluated as previously reported9 (Figure 2, Supplementary Appendix 3) for all frames. In brief, a strut was considered as covered if there was continuous tissue on the surface and both sides of the struts; coverage was further divided into protruding (D, strut protruding into the lumen) or embedded (E, F; continuous tissue, not interrupting the smooth lumen border). Otherwise, a strut was considered as uncovered (A-C). Strut malapposition was axial separation between the centre of the strut blooming artefact and the endoluminal surface >112 μm (BFS thickness). Strut neointimal thickness (NIT), lumen and stent areas were analysed every 1 mm. Neointimal area (NIA) was calculated as stent minus lumen area. Neointimal volume (NIV) and stent volume were calculated using Simpson’s rule and reported as total NIV, mean NIV (total NIV divided by length), and percent NIV (NIV divided by stent volume). Other OCT analyses were as previously described10.
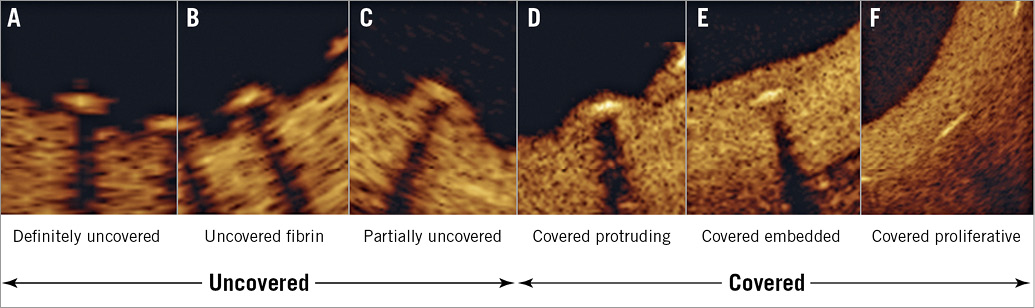
Figure 2. Six-category strut coverage classification with representative OCT images. A, B, C are classified as uncovered, and D, E, F are classified as covered (Supplementary Appendix 3 for details).
STUDY ENDPOINTS
The primary study endpoint was the degree of strut coverage as measured by OCT at nine months. Secondary study endpoints included: (i) median NIT, NIA, and NIV by OCT at different time points; (ii) in-stent LLL at nine months by QCA; and (iii) major adverse cardiac events (MACE) up to 12 months of clinical follow-up. The incidence of Academic Research Consortium-defined definite and probable stent thrombosis was monitored11.
DATA COLLECTION, MANAGEMENT AND ANALYSIS
All baseline and follow-up data were collected by the investigators with systematic verification by an independent study monitor (Supplementary Appendix 1). The study endpoint documentation was forwarded to a clinical research organisation and an independent clinical events committee (Supplementary Appendix 1) for adjudication of all deaths, myocardial infarction, stent thrombosis, and revascularisation.
STATISTICAL ANALYSIS
The study sample size was predefined at a minimum of 100 subjects without a formal hypothesis. Analysis of the primary endpoint was based on the intention-to-treat principle. Data are presented as mean±standard deviation or median with interquartile range (IQR) for continuous variables, and count with percent for categorical variables. Changes across the one to five months were compared using a completely randomised one-way analysis of variance with between-patient effects. Clustering of struts within the lesion and lesions within patients was accounted for by specifying a compound symmetric covariance structure in a hierarchical mixed model as random effect. Paired data were compared post hoc, using the Scheffé test. Changes between the first (one to five months) and the second (nine months) follow-up OCT, and between post-procedure and nine-month follow-up QCA were compared by linearly relating OCT or QCA variables to follow-up time and estimating a compound symmetric covariance with generalised estimating equation. The change in percent of covered struts as a function of the first follow-up as the log of days since stent implantation was modelled using a mixed effect. The homogeneity of variance of each time point was compared using the Brown-Forsythe test. Statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) or R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value <0.05 was considered statistically significant.
Results
STUDY POPULATION AND FOLLOW-UP COMPLIANCE
From December 2012 to July 2014, 106 patients were screened; 104 patients (110 de novo lesions) were enrolled (Figure 1). Three patients were subsequently excluded, one who died from a traumatic brain injury, one who developed renal failure, and another with emotional instability. Later, a fourth patient withdrew from the study. Thus, only 100 patients (96%) with 106 lesions (96.4%) completed the study. One third of the patients had diabetes, and approximately 10% presented with unstable coronary artery disease. The average age was 64±11 years, and 86% were male (Table 1).
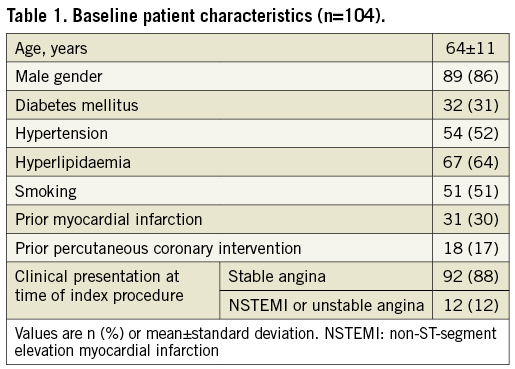
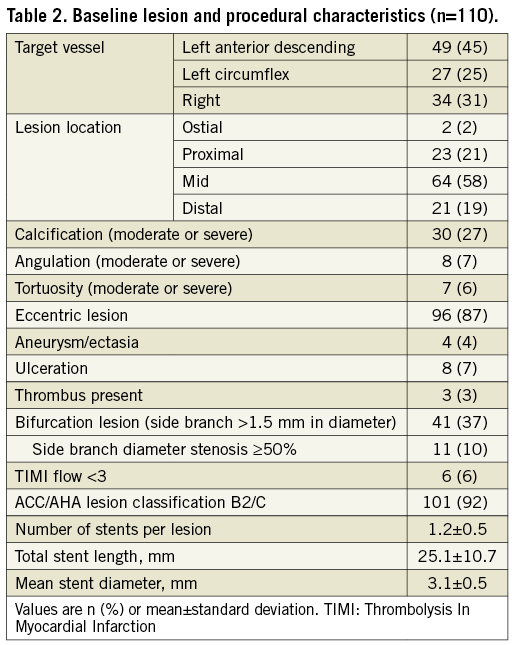
LESION AND PROCEDURAL CHARACTERISTICS
The majority of the lesions were class B2 or C with moderate to severe calcification (Table 2). During the index procedure, one lesion was treated in 94%, and two lesions in 6% of patients. An average of 1.2 stents per lesion were used; stent length was 25.1±10.7 mm, and the stent diameter was 3.1±0.5 mm.
QCA RESULTS
Mean lesion length was 22.4±10.2 mm (Table 3). Reference vessel diameter was 2.9±0.5 mm and was <2.7 mm in approximately one third of patients. Mean in-stent LLL at nine months was 0.21±0.30 mm.
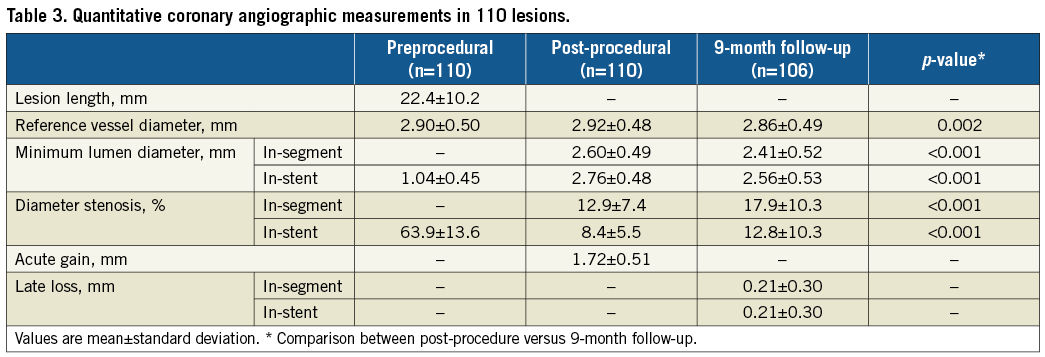
OCT RESULTS
Baseline post-procedural OCT showed good stent expansion (85.0±17.3%), and lipidic plaque behind stents was observed in 69% of lesions without differences among follow-up subgroups. There were no differences in baseline clinical, angiographic, procedural, and OCT findings among subgroups (Supplementary Table 1, Supplementary Table 2).
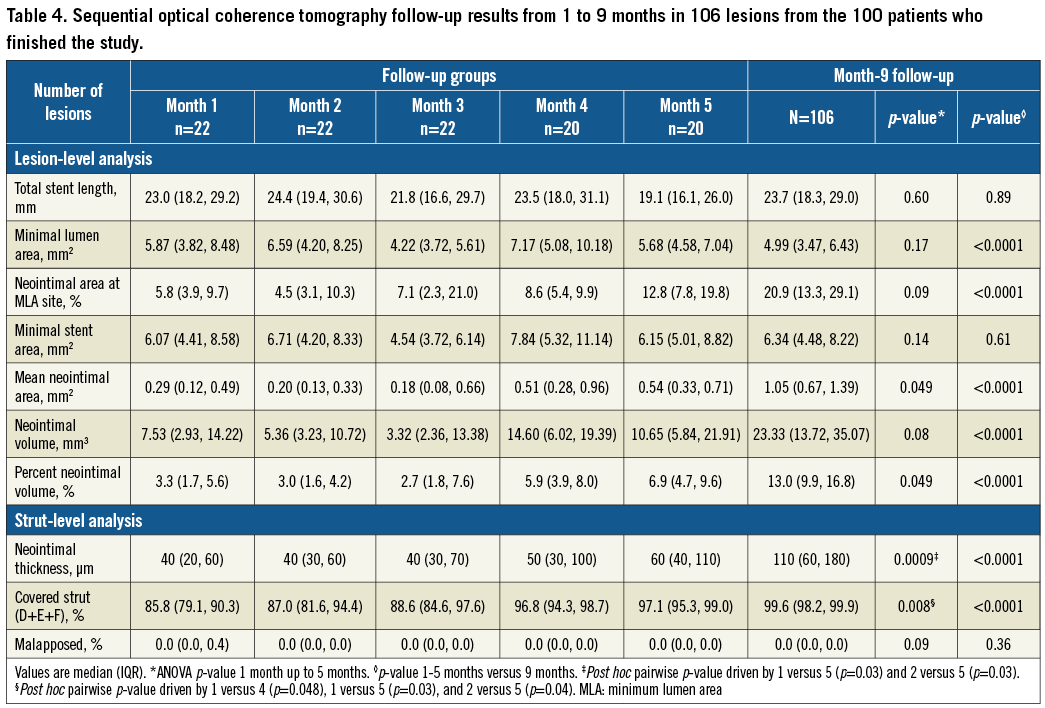
The early healing profile of the BFS is shown in Figure 3; the “worst” coverage in the one-month group was 48.2% and this increased to 87.1% at five months. The variability of coverage was wider in the early months. Changes in the prevalence of coverage categories are presented in Figure 4. At nine months, the median strut coverage reached 99.6% (IQR 98.2-99.9; range 85.4-100). NIT, NIA, and NIV all remained low at various time points (Table 4, Supplementary Table 3). Although NIV gradually increased by nine months, it remained low at only 13.0% (IQR 9.9-16.8), and the minimal lumen area (MLA) was well preserved at 4.99 mm2. Qualitatively, in the first five months, only 53% of 88 stented lesions had a quantifiable neointima (NIT >100 µm and ≥3 mm in length) with a homogeneous pattern. The presence of lipidic plaque behind the stent at baseline was associated with homogeneous neointima in the first five months (odds ratio [95% confidence interval] =3.7 [1.3, 10.6], p=0.01). Other covariates that were tested and had no association included stent expansion, total stent length, age, and diabetes mellitus. By nine months, neointimal homogeneity increased to 80%. One case showed a small organised thrombus at two months (persisting at nine months) without any clinical event, and another lesion showed lipidic plaque (neoatherosclerosis) at nine months. No late acquired evagination was observed.
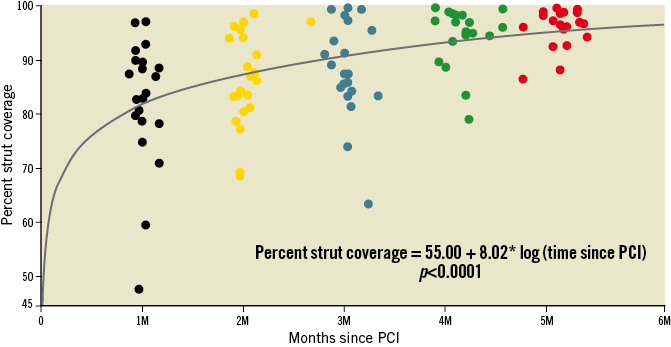
Figure 3. Early healing profile of the BioFreedom stent. Percent stent strut coverage (classified as D, E, F in Figure 2) with clustered individual results. Coverage progressively increased over time post PCI. The range of maximum and minimum coverage is worse at one month and improves over time (one month to five months, homogeneity of variance, p=0.005). Note: the section of curve from baseline to one month is interpolation.
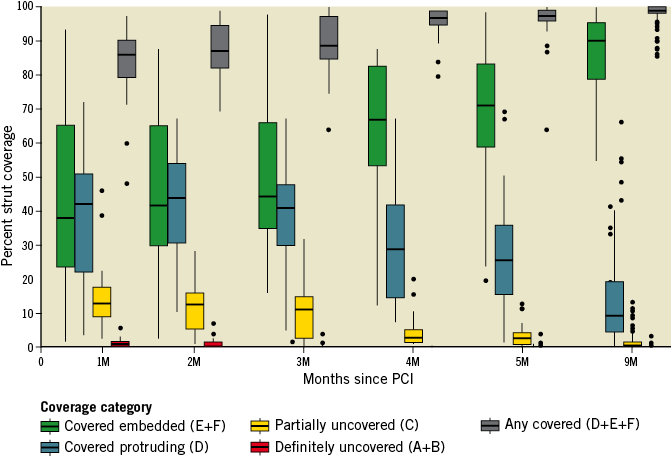
Figure 4. Changes in prevalence of coverage categories. The overall percent coverage (D, E, F, represented by the grey bar) increased progressively over time. Incomplete early strut coverage (A, B, C, D) was gradually replaced by more mature categories (E, F) at nine months.
SAFETY ANALYSIS
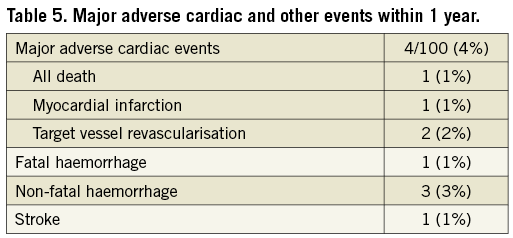
All adverse events up to one year are listed in Table 5. One patient died from a subdural haematoma after a fall. Another patient developed a non-STEMI at six months in a non-target vessel and was successfully treated by PCI. Major haemorrhage was observed in four patients, including the patient who died from a subdural haematoma. One patient reported left-sided numbness without motor deficit and was labelled as having a stroke; computed tomography scan was normal, and full recovery followed. Clinical restenosis requiring revascularisation by PCI occurred in two patients (2%) at nine months. No definite or probable stent thrombosis was recorded. The overall MACE rate was 4% (4/100).
Discussion
In this study, sequential OCT and angiographic examinations were conducted to characterise the early healing profile of the new polymer-free BFS. The OCT findings, in concordance with a QCA LLL of 0.21±0.30 mm at nine months, support a BFS antirestenotic efficacy similar to traditional DES12,13.
The primary objective of this study, however, was to establish a favourable early healing profile and subsequent neointimal transformation for the new polymer-free BFS.
OCT has an 80% sensitivity and 97% specificity for identifying strut coverage correctly14, but this refers to a more mature neointima beyond six months and may not be applicable to early coverage15. At early time points, stent struts may be covered by a thin but formed cellular neointimal layer, which may be overlooked by OCT, resulting in a false negative finding. Conversely, a thin layer of fibrin, not clearly discernible by OCT, may lead to an overestimation of coverage. OCT cannot identify true endothelium.
Bearing these limitations in mind and considering that this study lacks a control arm, caution is required when interpreting these OCT results relative to other reports of early strut coverage. In a 15 STEMI-patient cobalt-chromium bare metal stent study reported by Prati et al16, 89.1% coverage was described at three to seven days. Nishinari et al17 reported coverage of 70.4±12.6% at one month and 86.0±10.8% at two months in apposed struts in the Endeavor® zotarolimus-eluting stent (Medtronic, Minneapolis, MN, USA). Another polymer-free nano-sized-pore surface sirolimus-eluting stent (Nano™; Lepu Medical, Beijing, China) reported a median coverage of 93.0% (IQR 83.2-96.5) at three months18. The BFS used in the current study compares favourably with or exceeds these observations with a high median coverage of 85.8% (IQR 79.1-90.3) at one month, 87.0% (IQR 81.6-94.4) at two months, and 88.6% (IQR 84.6-97.6) at three months. At five months, coverage of 97.1% (IQR 95.3-99.0) is on a par with other DES at six months, including XIENCE V® (Abbott Vascular), PROMUS™ (Boston Scientific, Marlborough, MA, USA), and TAXUS™ Express™ (Boston Scientific)15. Finally, by nine months the nearly complete (99.6% [IQR 98.2- 99.9]) coverage is equivalent to the BioMatrix Flex™ with biodegradable polymer (Biosensors)19.
Currently, there is no consensus on the degree of early strut coverage allowing safe discontinuation of DAPT. Won et al20 proposed an uncovered strut threshold of 5.9%; however, OCT measurements were made at late time points between six and 12 months, and all patients received at least one-year DAPT. Because only six events were observed, two of them actually occurring during the full DAPT period, the recommendation does not apply to the early healing investigated in our study. Histologically, morphological stent coverage of <70% per section is highly associated with the risk of stent thrombosis21. Compared with histology, a threshold based purely on in vivo OCT coverage to define a DAPT duration sufficient to protect against stent thrombosis seems difficult.
Nevertheless, coverage observed over time, revealed by the healing curve using the six-category in vivo classification in this study, may represent valuable scientific evidence and prove useful for guiding optimal DAPT duration for a new device. For the BFS, coverage of 70% is achieved in less than one month (Figure 3), possibly predicting that one-month DAPT may prove clinically sensible with this new polymer-free stent, particularly in patients at high bleeding risk. This is in concordance with the favourable clinical outcomes (better safety in the BFS arm compared to the bare metal stent control) in the LEADERS FREE trial7, using one-month DAPT in a cohort of high bleeding risk patients. Moving forward, a future new stent platform advocating a shortened DAPT regimen should have its healing profile established by sequential OCT imaging before claiming safety.
Limitations
The major limitation is the absence of a control group. Also, the number of patients was relatively small, even though with the large amount of data generated by the longitudinal sequential protocol results were homogenous, solid, and in concordance with QCA findings at nine months. The current data apply only to patients with stable angina or NSTEMI/unstable angina and not to STEMI patients, and to patients who were treated with OCT guidance and with limited stent overlap.
Conclusions
Longitudinal sequential OCT assessments confirmed rapid healing and healthy neointimal transformation of the new polymer-free BFS and established, in concordance with angiographic QCA assessment, its antiproliferative efficacy at nine months. These benefits are concordant with the results of the recently published LEADERS FREE trial7.
| Impact on daily practice Rapid healing of the BioFreedom stent with favourable nine-month neointimal transformation strengthened the rationale of using the BioFreedom stent with shortened DAPT duration in high bleeding risk patients while maintaining the benefit of DES. |
Acknowledgements
The authors thank Rodolphe Ruffy, MD, for his kind assistance in the preparation of the manuscript.
Funding
This was an investigator-initiated study proposed and conducted by members of the Cardiology Division at Queen Mary Hospital, University of Hong Kong. BioFreedom stents were supplied by Biosensors Interventional Technologies. OCT catheters, core laboratory expenses, and monitoring and CEC costs were supported by an unrestricted grant from Biosensors International to the Study Centre.
Conflict of interest statement
S. Lee reports non-financial support (study stents) from Biosensors during the conduct of the study. R. Costa reports personal fees from Biosensors SA outside the submitted work. H. Stoll reports being a full-time employee of Biosensors International. A. Maehara reports grants from Abbott Vascular and Boston Scientific outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Independent study teams.
Supplementary Appendix 2. Quantitative coronary angiographic definitions.
Supplementary Appendix 3. Morphologic OCT classification of strut coverage.
Supplementary Table 1. Baseline patient characteristics stratified by interim follow-up subgroups in 100 patients with follow-up OCT.
Supplementary Table 2. Baseline angiographic, procedural, and OCT findings stratified by interim follow-up subgroups in 106 lesions with follow-up OCT.
Supplementary Table 3. Sequential optical coherence tomography follow-up results from 1 to 9 months in 106 lesions from the 100 patients who finished the study.
To read the full content of this article, please download the PDF.

