Bioresorbable vascular scaffolds (BVS) have the potential to revolutionise interventional cardiology practice1. The prophecy of a device that does the job, namely in providing temporary scaffolding to seal dissections and prevention of vessel recoil, elution of an antiproliferative drug to limit the healing response and then disappears to allow for the restoration of physiological and vasomotor function of the vessel, has universal appeal2-6. At present the Absorb everolimus-eluting BVS (Abbott Vascular, Santa Clara, CA, USA) is the only device that has entered clinical practice7-11, with the promise of multiple other devices currently in development12.
Although long-term evidence from appropriately powered randomised trials is awaited, there is a growing volume of smaller studies, registry and bench data supporting the use of the Absorb BVS in differing clinical presentations and lesion types – including acute coronary syndrome and ST-elevation myocardial infarction, small vessels, long lesions, ostial lesions, chronic total occlusions, calcified vessels, and coronary bifurcations7-11,13-18. To this expanding body of evidence, Ishibashi et al19 present important preliminary findings from the ABSORB EXTEND single-arm study7,20 relating to the deliverability of the Absorb device and some of the first reported cases relating to scaffold thrombosis.
Absorb device
The Absorb BVS is a balloon-expandable bioresorbable scaffold constructed of poly-L-lactide acid (PLLA) and coated with a bioresorbable poly-D, L-lactide that contains and controls the release of the antiproliferative drug everolimus. Approximately 80% of the drug is released within 30 days after implantation, and the remainder of the drug within four months. Based on studies in a porcine model, the Absorb BVS bioresorption process has been shown to commence at six months, with the expected loss of structural integrity and potential restoration of vasomotor function of the treated vessel at one year, and resultant completion of the bioresorption process at two to three years2-6.
Absorb BVS strut thickness
Due to the limited distensibility of the Absorb BVS polymeric platforms, various design iterations have been made to improve its radial strength to allow it to match its metallic counterparts, and to increase the distensibility of the device to allow for more flexibility in device expansion during deployment to reduce the possibility of device fracture during implantation2-6,20. Importantly, these have included the need for thicker struts. At the time of implantation, the total thickness of the Absorb BVS polymeric strut is approximately 156 μm, a value very similar to that seen with first-generation metallic drug-eluting stents.
Although Ishibashi et al state that the material the Absorb BVS is manufactured from (PLLA) has in vitro been shown to be “somewhat less thrombogenic than a metal without a coating”, they also accept that the presence of thicker Absorb BVS struts (156 microns) create alterations of shear stress (high shear stress on top of the strut and low shear stress behind the strut21,22) which may predispose to the triggering of platelet aggregation20,21,23. Provocatively, one may therefore hypothesise that the thicker struts of the Absorb BVS may place it at increased risk of scaffold thrombosis compared to new-generation thinner-strut drug-eluting stents, particularly if the Absorb BVS is overlapped (strut thickness >300 microns). In addition, porcine studies14,15 have shown that healing (neointimal coverage) of overlapping Absorb BVS is delayed at 30 days (equivalent to approximately six months in humans), with healing completed at 90 days (equivalent to approximately 18 months in humans). Consequently, determining the exact duration of dual antiplatelet therapy (DAPT) following Absorb BVS implantation, particularly when overlapped (Figure 1), is currently unknown. Furthermore, genetic variability in platelet reactivity to clopidogrel (high platelet reactivity) has been associated with adverse outcomes in subjects implanted with metallic drug-eluting stents24. In patients implanted with the Absorb BVS, it would therefore appear sensible either to increase the use of platelet reactivity testing, or administer newer antiplatelet agents where genetic variability in platelet reactivity poses less of a problem.
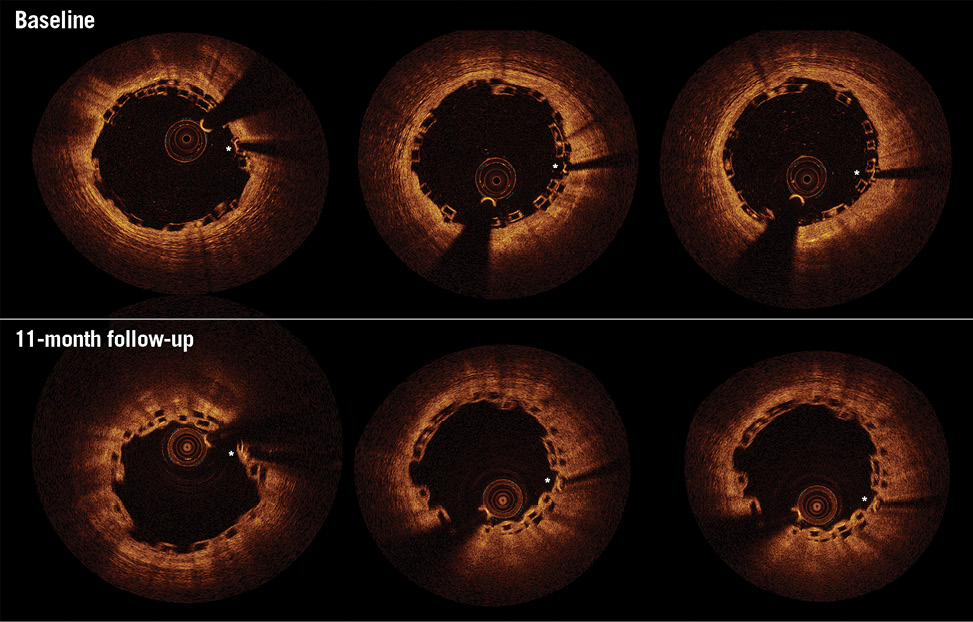
Figure 1. Optical coherence tomography (OCT) 11-month follow-up of overlapping Absorb bioresorbable vascular scaffolds (BVS). Matched baseline (upper) and 11-month (lower) OCT images of overlapping Absorb BVS implanted in the proximal left anterior descending artery (LAD) vessel. Asterisks indicate platinum markers of corresponding baseline and follow-up overlapping Absorb BVS. Eleven months following overlapping Absorb BVS implantation, the patient returned with unstable angina. Coronary angiography indicated the presentation was unrelated to the index procedure with a de novo lesion in the apical LAD. OCT imaging of the proximal LAD demonstrated the healing process to be incomplete at the overlapping Absorb BVS, with a lack of a smooth luminal contour and multiple uncovered overlapping struts (using a 30 μm threshold for coverage of the Absorb BVS strut from the endoluminal light backscattering strut boundary15,35). In light of these findings, the patient’s dual antiplatelet therapy was extended from one to two years. Courtesy of Manchester Heart Centre, Manchester Royal Infirmary, United Kingdom.
Deliverability of the Absorb device
As Ishibashi et al describe, because of its thicker struts, the Absorb BVS has a greater crossing profile (1.4 mm) compared to contemporary metallic stents, and a similar crossing profile compared to the first-generation (CYPHER®; Cordis, Johnson & Johnson, Warren, NJ, USA) drug-eluting stents. As a result, the Absorb BVS has been reported to cause friction between the device and tortuous/calcified vessels or the collar of a GuideLiner™ guide catheter extension system (Vascular Solutions Inc., Minneapolis, MN, USA) (Figure 2), with risk of scaffold dislodgement from the deploying balloon. It should however be emphasised that in over 80% of Absorb BVS cases no ancillary devices are required to allow its delivery7, with the following cascade of recommendations generally accepted to allow delivery of the Absorb BVS in more difficult cases:
1) Simple measures: use of an appropriately selected upfront guide catheter size and type to maximise back-up support; use of buddy wires.
2) Adequate lesion preparation, particularly in calcified disease: cutting/scoring balloons and rotational atherectomy.
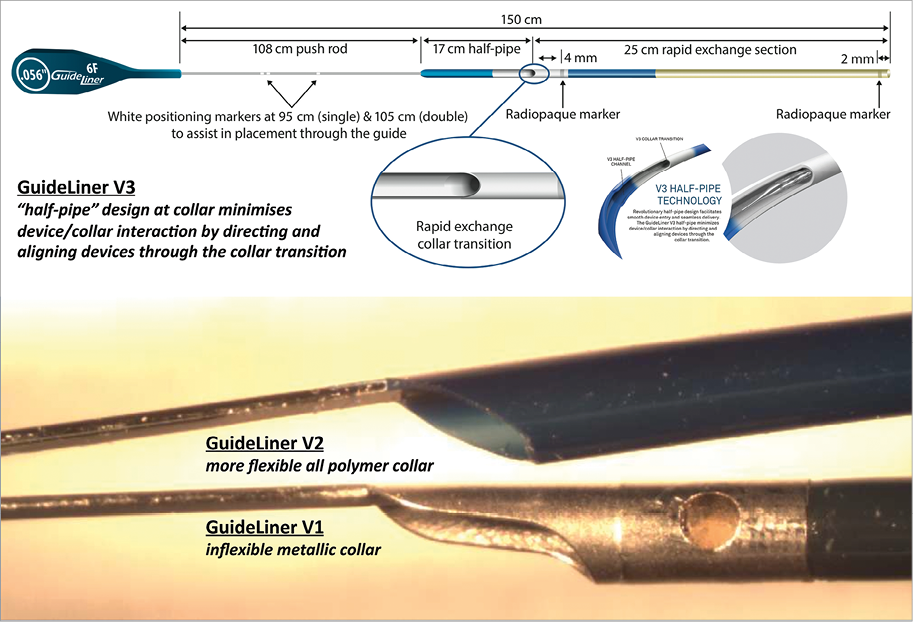
Figure 2. Evolution in the design of the GuideLiner extension system. The GuideLiner guide catheter extension system has gone through several design modifications to minimise the issue of the device/collar interaction where devices may potentially dislodge and deform. These have ranged from moving from an inflexible metallic collar (V1) to a more flexible all polymer collar (V2), to the more recent “half-pipe” design at the collar (V3) as detailed above. In addition, the actual guide catheter extension system (rapid exchange section) was lengthened from 20 cm in V1 to 25 cm in versions two and three to allow deeper intubation into coronary vessels and bypass grafts, and to prevent loss of coaxial alignment of the collar to the guide catheter (and increased risk of device/collar interaction), which may occur in secondary curves of certain guide catheters such as the Amplatz27,29,31,32.
Notably, following the anecdotal case of Absorb BVS dislodgement with the GuideLiner guide catheter extension system in the current study19, the authors advise against the use of the GuideLiner to aid Absorb BVS delivery. Guide catheter extension systems include the over-the-wire Heartrail® (Terumo Corp., Tokyo, Japan) and the easier to use monorail rapid exchange GuideLiner systems (Vascular Solutions). Such systems have proven to be extremely versatile in making intervention achievable in the most challenging anatomies25-30. The atraumatic and soft tip of guide catheter extension systems has facilitated very deep intubation into coronary vessels and bypass grafts, thereby improving back-up support and allowing the bypassing of proximal points of obstruction, such as tortuosity and calcification, to aid the delivery of the stent more distally.
The GuideLiner system incorporates a collar to allow for a monorail rapid exchange system. As well as the Absorb BVS19, metallic stents have been reported to become damaged or dislodged at this collar interface27,31,32. Various design modifications have subsequently been made to the collar to minimise this issue (Figure 2).
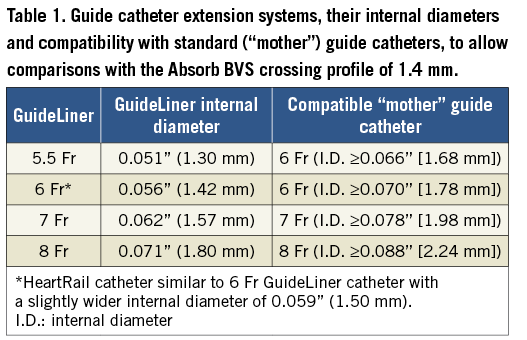
Despite the manufacturer’s guidance for the Absorb BVS not to be used with 5-in-6 or 6-in-7 guide catheter extension systems “as doing so will result in an inner diameter that is too small for use with the Absorb BVS system”33, 6 Fr and 7 Fr guide catheter extension systems have inner diameters that exceed the crossing profile of the Absorb BVS (1.4 mm) (Table 1), and have been reported to allow delivery of a 3 mm Absorb BVS11,34.
Consequently, understanding the versatility and limitations of guide catheter extension systems may actually facilitate delivery of the Absorb BVS, especially in very tortuous and calcified vessels as reported in the literature11,34. Moreover, given the recommendations not to reinsert the Absorb BVS after one attempt at delivery, in order to avoid prolonged contact with moisture and the consequent increased risk of device dislodgement19, perhaps it would be prudent to try to maximise the chances of delivering the Absorb device through challenging anatomy using a guide catheter extension system – particularly when problems are expected in delivery despite appropriate adjunctive interventional techniques.
Conclusion
As our experience of the Absorb and other bioresorbable devices continues to evolve, it is clear that many more lessons remain to be learned in order for us to realise fully the potential long-term benefits of this innovative technology.
Conflict of interest statement
The authors have no conflicts of interest to declare.

