Bioresorbable vascular scaffold (BVS) use in clinical practice for the treatment of coronary lesions has expanded rapidly since they became commercially available. Recently, reports of clinical outcomes with BVS in a broader, more complex “real-world” patient population have started to emerge1-3. In a multicentre retrospective registry published recently in EuroIntervention, Capodanno and colleagues1 studied 1,189 patients in whom the Absorb BVS (Abbott Vascular, Santa Clara, CA, USA) was used in routine practice (GHOST-EU registry). They found a target lesion failure (TLF) rate of 4.4% at six months, and the incidence of definite/probable scaffold thrombosis was substantial, with rates of 1.5% at 30 days and 2.1% at six months1, higher than that typically observed with second-generation DES.
How to interpret these data and are there reasonable grounds for concern?
In the ABSORB II trial, target vessel myocardial infarction was identified in 4.2% of the Absorb group versus 1.2% of the XIENCE group (p=0.07). Probable and definite stent thrombosis was detected in three patients in the BVS group (0.9%) versus no thrombosis in the XIENCE group (0%) at one year4. In less complex patients, such as those in the ABSORB cohort A and cohort B trial, no scaffold or late scaffold thrombosis was observed during follow-up (up to five years for cohort A)5. Similarly, in the first 450 patients enrolled in the ABSORB EXTEND study, the rate of subacute or late scaffold thrombosis was only 0.89% at 12 months3.
Scaffold thrombosis is still a new phenomenon and remains a matter of debate6,7. Upcoming data from all-comers studies, longer-term follow-up and large trials including the ABSORB III and ABSORB EXTEND will probably shed light on this question.
Impact of strut thickness
The Absorb BVS scaffold is based on a poly-L-lactic acid (PLLA) backbone with a 3 μm surface drug coating giving a total strut thickness of 156 μm. In comparison, the metallic XIENCE PRIME DES stent (Abbott Vascular) is based on an 81 μm cobalt-chromium alloy with a fluorinated polymer coating leading to a total strut thickness of ~97 μm.
Strut size is an important parameter that affects healing response after metal stent implantation, and insights from preclinical and clinical studies have shown that thick metal struts have delayed re-endothelialisation and elicit more neointimal proliferation than a thinner strut design8-11. Strut coverage has been shown to be influenced by shear stress patterns on the stent strut surface8,12,13. In addition, thick metal struts have also been shown to have more thrombogenicity than thin-strut stents when tested using an ex vivo perfusion model8.
So does the same effect extend to bioabsorbable scaffolds?
Simulations of flow profile around BVS struts compared with those of a current metallic stent (BVS=156 µm and XIENCE=97 µm) clearly show that local flow patterns are affected by strut thickness (Figure 1). It is therefore reasonable to assume that strut thickness is going to have a similar impact as in a metallic stent, at least until the scaffold starts to resorb. However, evidence is still lacking and not all principles can be applied in the same way to biodegradable scaffolds: for example, neointimal hyperplasia affects biodegradable scaffolds in a similar way to metallic stents, with large struts producing more neointimal response than thinner struts10,14. However, long-term healing response with biodegradable technology is markedly different from that of metal stents: vessel remodelling continues as the scaffold dismantles, with late compensatory remodelling preserving against the loss of lumen area10,14.
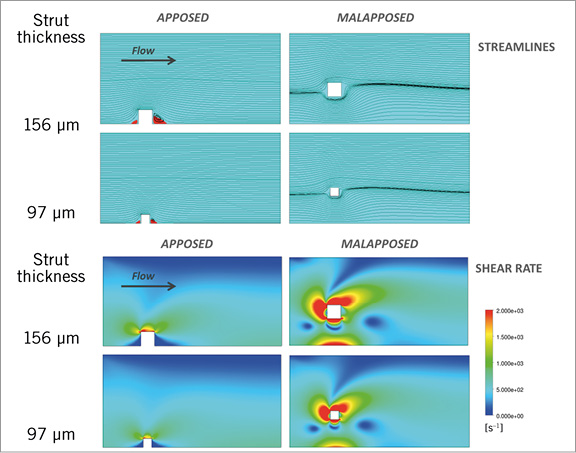
Figure 1. Impact of strut thickness on blood flow profiles for a model simulating cases of well-apposed and malapposed struts. Simulated blood flow velocity profiles (top panel) for different cases of strut apposition: apposed (left) and malapposed (right) (strut to wall distance =300 µm). Models are representative of a 3 mm diameter straight coronary artery flow with a parabolic upstream velocity profile and a peak velocity of 50 cm/s. The two strut thicknesses considered correspond to a total strut thickness (strut+coating) of 156 µm (BVS) and 97 µm (XIENCE). The corresponding shear rate profile in blood around the stent strut simulated for each case (lower panels) shows that flow disturbances and high shear rates (red) are increased primarily by strut malapposition.
In addition to strut size, other parameters such as incomplete strut apposition (ISA) also affect flow profile: immediately after implantation, incompletely apposed struts act as obstacles which disrupt the laminar flow and create an area of high shear rate8,12. The further away the malapposed strut is from the wall, the larger the magnitude of shear rate disturbances around the strut8,12. A high shear rate (>1,000 s–1) activates platelets in a dose-dependent manner through the von Willebrand factor binding to glycoprotein (GP) Ib and GP IIb/IIIa receptors15. Multiple clinical, pathological and model studies have exposed the association between ISA and thrombosis8,16-18. However, the relative impact of strut thickness versus malapposition on the risk of thrombosis is not yet fully known and practically impossible to evaluate directly in a clinical study.
Comparison of flow patterns for different strut thicknesses in both apposed and malapposed cases shows that strut thickness has a relatively smaller impact on flow disturbances as compared to stent malapposition (Figure 1, Figure 2). The influence of malapposition on high shear disturbances is several times more important than the effect of strut dimension.
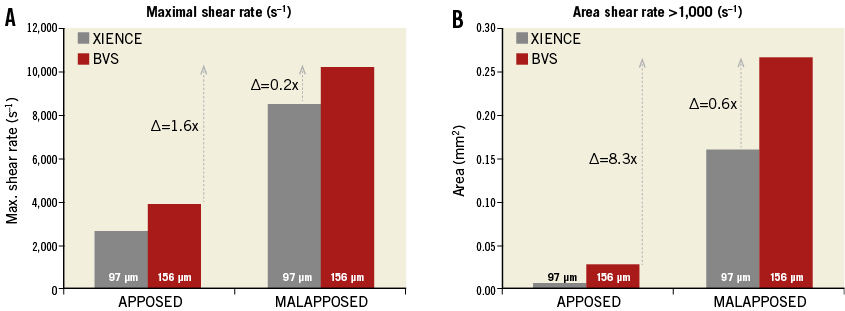
Figure 2. Maximal shear rate (A) and area of shear rate >1,000 s–1 (B). Comparison of the shear rate values computed for the two strut thicknesses shows that strut thickness has a relatively lesser impact on high shear flow disturbances as compared to strut malapposition. High shear rate activates platelets in a dose-dependent manner through the von Willebrand factor binding to glycoprotein (GP) Ib and GP IIb/IIIa receptors15.
Currently, bioabsorbable scaffolds require a large strut thickness to provide sufficient radial support and prevent acute and late elastic recoil. The principle of “the thinner the better” only applies as long as the radial force can be maintained. Biodegradable polymers have inherent limitations in terms of mechanical strength and, while manufacturing processes can improve strength, achieving a radial force comparable to a metallic platform still currently requires a substantial strut thickness, typically >150 µm.
However, if flow disturbances and high shear rates are increased primarily by strut malapposition (Figure 1), should we not focus on ensuring complete scaffold apposition and strut embedding in the vessel wall rather than reflecting on BVS strut thickness?
Conflict of interest statement
The authors have no conflicts of interest to declare.

