
Abstract
Aims: The safety of bioresorbable scaffolds (BRS) has recently been challenged. However, it is unclear whether outcomes depend on the complexity of the lesion or on the technique used to implant the device. The aim of this study was to report on the outcomes after BRS implantation in complex lesions.
Methods and results: This investigator-initiated, single-centre, single-arm observational study recruited 657 consecutive patients (79% male, 66.7% acute coronary syndrome, age 63±12 years). Three hundred and twenty-two lesions (42.3%) in 297 (45.2%) patients with type B2 or C lesions were classified as the “complex lesions group”. Post-procedural residual stenosis was slightly but significantly greater in the complex lesions group (15.7±11.3% vs. 13.5±10.2%, p=0.0109). The median follow-up was 1,076 (762-1,206) days without difference between groups. The Kaplan-Meier rates of early scaffold thrombosis (3.5% vs. 1.1%, p=0.0478, HR 3.03 [1.06-8.70]) and scaffold restenosis (9.9% vs. 9.1%, p=0.0262, HR 2.34 [1.11-4.94]) were higher in patients with complex lesions than in those with simple lesions. Late/very late thrombosis, death, repeat myocardial infarction, or repeat coronary interventions were not different. In patients in whom strict guidelines for implantation were applied, the incidence of thrombosis was reduced by 76% in complex lesions and by 92% in simple ones, such that there were no differences between groups (2.3% vs. 0.5%, p=0.3899). In contrast, the incidence of scaffold restenosis was reduced by 59% and 89%, and a difference between groups persisted (7.0% vs. 1.6%, p=0.0235).
Conclusions: BRS implantation in complex lesions is, as expected, associated with higher incidence of events as compared to simple ones. The technique used at the time of the implantation, however, reduces the incidence of adverse outcomes.
Abbreviations
AHA: American Heart Association
BRS: bioresorbable vascular scaffold
CABG: coronary artery bypass graft
CI: confidence interval
CTO: chronic total occlusion
DES: drug-eluting stent
eGFR: estimated glomerular filtration rate
HR: hazard ratio
IQR: interquartile range
LAD: left anterior descending
LCX: left circumflex
LM: left main stem
LVEF: left ventricular ejection fraction
MI: myocardial infarction
NSTEMI: non-ST-elevation myocardial infarction
PCI : percutaneous coronary intervention
QCA: quantitative coronary analysis
RCA: right coronary artery
RVD: reference vessel diameter
ScR: scaffold restenosis
ScT: scaffold thrombosis
STEMI: ST-elevation myocardial infarction
TIA: transient ischaemic attack
TLF: target lesion failure
TLR: target lesion revascularisation
Introduction
Coronary bioresorbable scaffolds (BRS) were introduced in 2012 for the treatment of de novo atherosclerosis in native coronaries without restriction regarding the type or complexity of the lesions. Although initial data from registries and randomised trials were limited to type A lesions, the unrestricted approval rapidly led to the use of scaffolds in more complex settings including ostial lesions1, bifurcation lesions2,3, long lesions4, chronic total occlusions5,6, patients with diabetes4,7 or those presenting with ACS8. As compared to type A lesions, treatment of patients with complex lesions is associated with suboptimal device deployment, an increased rate of incomplete revascularisation, and an increased rate of subsequent events, especially when combined with diabetes9. As for the mechanisms of this observation, an incomplete device expansion after treatment of calcific plaques has been associated with both early and late device failure10. Also, specifically for BRS, the risk of recoil might be higher in lesions with a fibronecrotic component, which might in turn lead to late failure when bioresorption processes progress11.
Although experiences describing the short-term and midterm feasibility in complex lesions as a whole and in the above individual settings have already been published12,13, it remains unexplored whether BRS implantation in complex settings, as compared to simple lesions, leads to increased rates of complications over long-term follow-up periods. In addition, it is not known whether the introduction of particular care at the time of implantation might significantly reduce the rate of complications.
Methods
STUDY DESIGN
Consecutive patients who underwent percutaneous coronary intervention from May 2012 to December 2014 in the catheterisation laboratory of the University Medical Center Mainz with at least one BRS (Absorb™; Abbott Vascular, Santa Clara, CA, USA) were enrolled in this registry. Lesions were classified according to the American Heart Association classification14. The outcomes of patients with lesion(s) presenting two or more type B criteria or type C lesion(s) (the “complex lesions group”) were compared to those with simple lesions (type A or one type B criterion).
The study is part of the MICAT project (NCT02180178), which was approved by the local ethics committee.
BRS IMPLANTATION
Details on the inclusion criteria and patient/lesion selection have already been published elsewhere15 and are shown in the Supplementary Appendix.
QUANTITATIVE CORONARY ANALYSIS
Quantitative coronary analysis (QCA) was performed with Xcelera, R 4.1 (Philips, Amsterdam, the Netherlands) in the local laboratory. Key measurements (before and after implantation) included the interpolated reference vessel diameter (RVD) and the (in-BRS) minimum lumen diameter (MLD). Residual stenosis was calculated as (RVD−MLD)/RVD.
Lumen gain was calculated as post-procedural MLD−preprocedural MLD.
Reproducibility and repeatability data for our laboratory have been described previously15.
ENDPOINTS
Follow-up was performed by trained personnel by computer-assisted telephone interview as previously published15. Events were adjudicated after review of the original clinical data by two interventional cardiologists according to the Academic Research Consortium definitions16 (Supplementary Appendix).
STATISTICAL ANALYSIS
Continuous data are described as mean and standard deviation or median and interquartile range based on the analysis of Q-Q plots. Parametric or non-parametric tests were used accordingly. Categorical data are described as total numbers and proportions and were analysed with the χ2 test or Fisher’s exact test.
Cox regression analysis was used to describe the association between clinical/procedural parameters and outcome events. For both endpoints that were associated with complex lesions (early ScT and ScR beyond 365 days), a propensity score was built to remove potential treatment assignment bias. Clinical variables associated with either lesion type or the outcome (ScT and ScR) were used to calculate the inverse probability of treatment weights. A list of the variables and the results of the propensity weighting are presented in the Supplementary Appendix.
Survival curves are presented as Kaplan-Meier curves with corresponding log-rank p-values.
Given their importance in determining patient outcomes after BRS implantation, the role of the following procedures was assessed retrospectively (“optimal implantation technique”):
1)predilatation with a balloon of the same nominal size as the BRS;
2)vessel size (interpolated RVD, measured at the end of the procedure) comprised between 2.5 and 3.5 mm;
3)BRS sizing: implantation of a BRS of the same size as the reference vessel diameter (nominal diameter to RVD ratio comprised between 0.9 and 1.1);
4)post-dilatation at 14-16 atm with non-compliant balloons of the same size or 0.5 mm larger than the BRS OR (given the high prevalence of thrombotic lesions in the complex lesions group) achievement of an optimal final result independently of post-dilatation (residual stenosis <20% and ratio of final MLD to nominal BRS size >80%).
Separate outcome analyses were performed for events which occurred within 30 days after implantation, between one month and one year, and three years after implantation. All analyses should be considered exploratory. Data were analysed with MedCalc, Version 9.2.1.0 (MedCalc Software, Ostend, Belgium) or R (R Foundation for Statistical Computing, Vienna, Austria).
Results
PATIENT CHARACTERISTICS
Six hundred and fifty-seven patients with 762 lesions were included in this registry. Of these, 297 (45.2%) patients had at least one complex lesion treated with a BRS, for a total of 322 complex lesions (42.3%) treated. Table 1 shows the patient characteristics: 79.0% of the patients were male (complex lesions group versus simple lesions group: 76.8% vs. 80.8%, p=1.00). The mean age of the population was 63±12 years (62±12 vs. 64±12, p=0.0471). The prevalence of hypertension (68.0% vs. 76.7%, p=0.0168) was higher in the simple lesions group, while other risk factors were equally distributed.
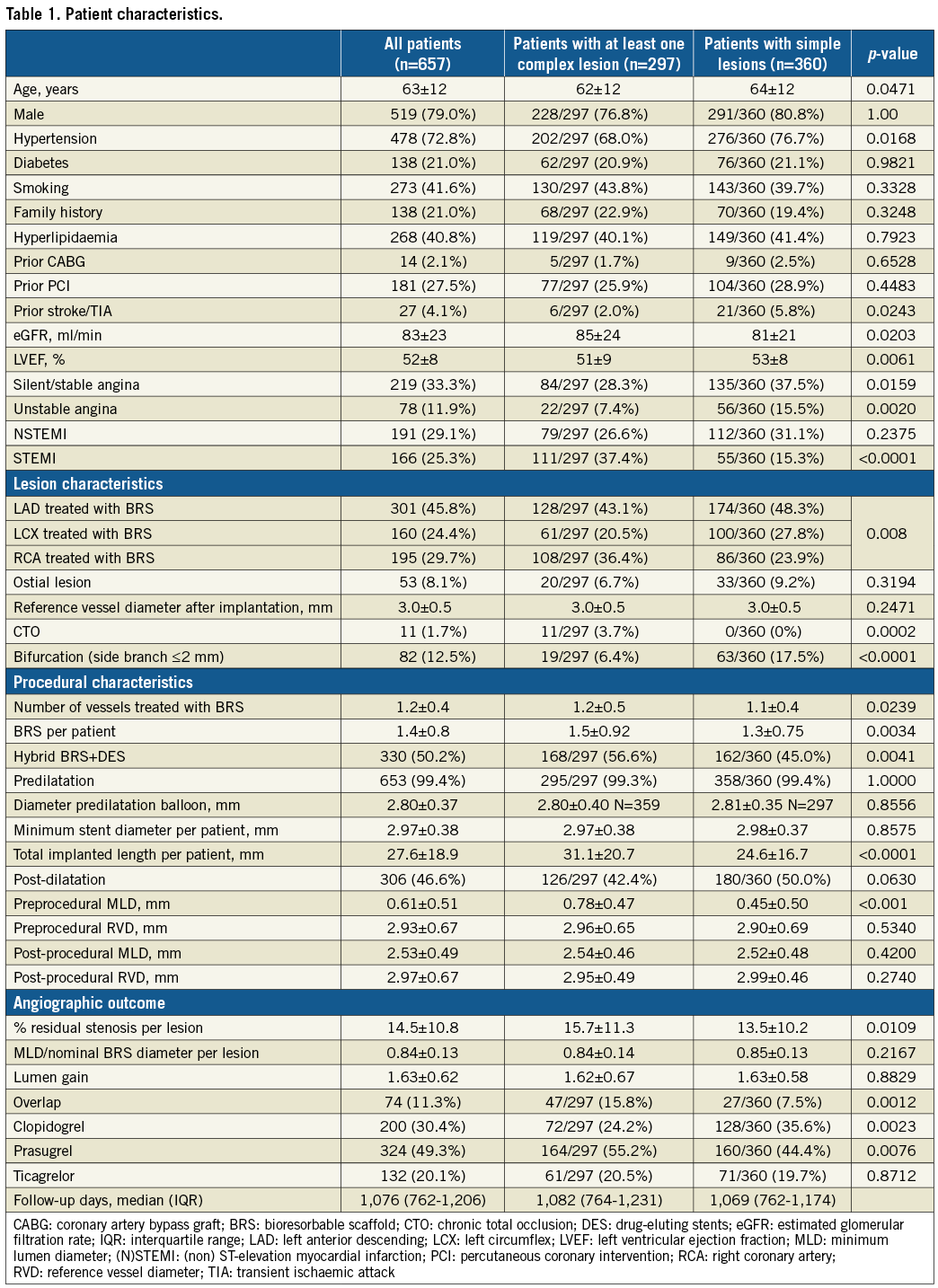
There was no significant difference in the prevalence of prior bypass surgery (1.7% vs. 2.5%) and prior PCI (25.9% vs. 28.9%); in contrast, a history of previous stroke or transient ischaemic attack was more frequent in the simple lesions group (2.0% vs. 5.8%, p=0.0243). Patients with complex lesion(s) had a significantly higher estimated glomerular filtration rate (eGFR) and lower left ventricular ejection fraction (85±24 mL/min vs. 81±21 mL/min, p=0.0203, and 51±9% vs. 53±8%, p=0.0061, respectively).
The initial presentation of the patients differed significantly between the two groups (silent/stable angina 28.3% vs. 37.5%, p=0.0159; unstable angina 7.4% vs. 15.5%, p=0.0020; NSTEMI 26.6% vs. 31.1%, p=0.2375; STEMI 37.4% vs. 15.3%, p<0.0001, for complex and simple lesions, respectively).
LESION CHARACTERISTICS
Features determining the complexity of the lesions included chronic total occlusion (3.7%), acute thrombotic occlusion (25.3%), a lesion >20 mm in length (49.4%) and a combination of at least two factors from among length comprising between 10 and 20 mm, moderate calcification and/or tortuosity, intravascular thrombus, and ostial lesions (47.4%). Preprocedural RVD was not different between groups (complex: 2.90±0.69 mm; simple: 2.96±0.65 mm, p=0.420). In contrast, the MLD was smaller (0.45±0.50 vs. 0.78±0.47 mm, p<0.001), and therefore the % stenosis was greater (83±19% vs. 73±15%, p<0.001) in complex lesions.
The prevalence of multivessel disease was also higher (number of vessels treated 1.2±0.5 vs. 1.1±0.4, p=0.0239), and the total stented length per lesion was greater (26±15 mm vs. 22±11 mm, p<0.0001) in the complex lesions group. The number of scaffolds implanted per patient (1.5±0.9 vs. 1.3±0.8, p=0.0034), the total BRS length per patient (31±21 mm vs. 25±17 mm, p<0.0001), and the number of overlapping BRS (15.8% vs. 7.5%, p=0.0012) were higher in the complex lesions group. Hybrid metallic and BRS stenting was more frequent in complex lesions (56.6% vs. 45.0%, p=0.0041). Ostial lesions were present in 8.1% (6.7% vs. 9.2%, p=0.3194).
Although bifurcation lesions to be treated with a two-stent technique were excluded a priori, lesions with side branches ~2 mm in diameter in which a provisional strategy was applied were included. These were less frequent in the complex lesions group (6.4% vs. 17.5%, p<0.0001). The (post-procedural) RVD was smaller than 2.5 mm in 6.8% of the lesions in the simple versus 8.4% of the lesions in the complex lesions group (p=0.5046). “Simple” (i.e., non-occlusive, <10 mm, non-calcific, etc.) thrombotic lesions were present in the simple lesions group.
LESION TREATMENT AND IMMEDIATE ANGIOGRAPHIC RESULTS
Predilatation was used in virtually all lesions independently of the lesion complexity (99.3% vs. 99.4%, p=1.00). Post-dilatation was performed in 42.4% vs. 50.0% (p=0.0630) of the patients. The pressure used was 15.0±2.6 vs. 14.4±2.9 atm (p=0.143), for the complex and simple lesions group, respectively.
The ratio of MLD to nominal BRS diameter, expressing BRS deployment, was 0.8±0.1 vs. 0.8±0.1 (p=0.2167), for the complex and simple lesions group, respectively. All implantation criteria described above (“optimal technique”) were respected in 170 patients (53%) in the complex lesions group and 253 (57.5%) patients in the simple lesions group (p=0.2416).
After intervention, RVD and MLD were not different between groups (RVD, complex: 2.99±0.46 mm vs. simple: 2.95±0.49 mm, p=0.549; MLD: 2.52±0.48 vs. 2.54±0.46 mm, p=0.534). Acute lumen gain was also not different (complex: 1.62±0.67 mm vs. simple: 1.63±0.58 mm, p=0.8829), while the % residual stenosis was slightly greater in the complex lesions group (15.7±11.3% vs. 13.5±10.2%, p=0.0109).
FOLLOW-UP
The median follow-up was 1,076 (762-1,206) days (p=0.7430 between groups). A complete three-year follow-up was available in 386 of 416 (93%) eligible patients (p=0.3345 between groups). Table 2 and Supplementary Table 1 show the number of events, the crude incidence and Kaplan-Meier estimates of the endpoints and the hazard ratios in patients with and without complex lesions at each time point.
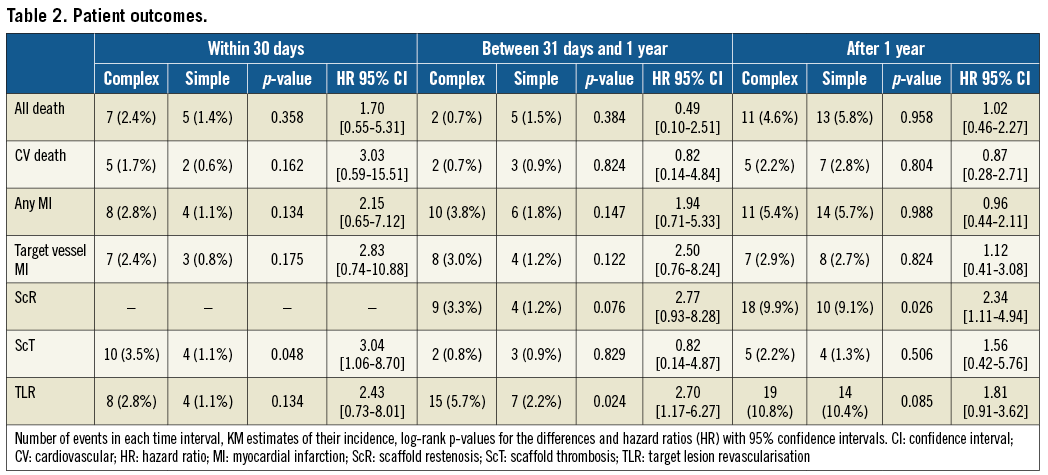
Figure 1 and Figure 2 depict the Kaplan-Meier survival curves of ScT and scaffold restenosis (ScR).
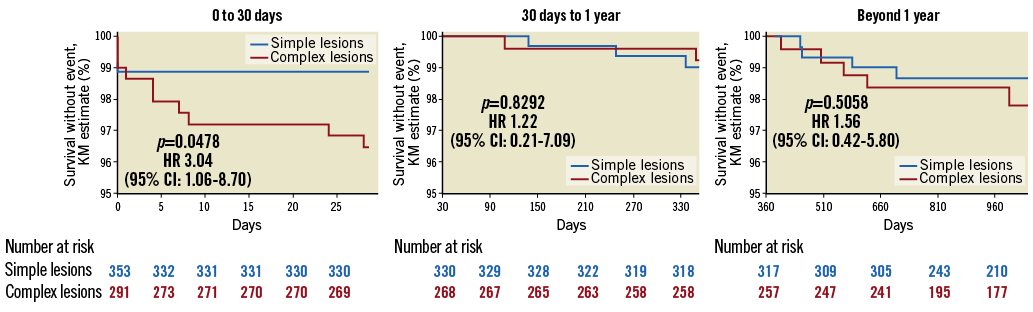
Figure 1. Incidence of scaffold thrombosis according to lesion complexity. The incidence of early ScT was higher in the complex lesions group, and a trend was manifest for following time points.
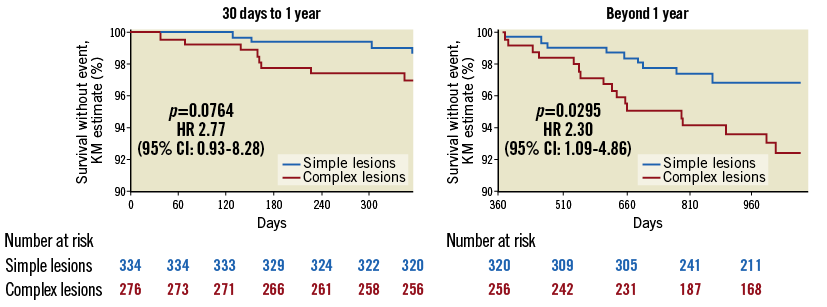
Figure 2. Incidence of scaffold restenosis according to lesion complexity. The incidence of ScR was higher in complex lesions.
The incidence of early (<30 days) ScT and that of ScR beyond one year after implantation was significantly higher in patients with complex lesions (respectively, 3.55% vs. 1.1%, p=0.0478, and 9.9% vs. 9.1%, p=0.0262). These associations were confirmed in the propensity score analysis (ScR: lesion-level analysis after average treatment effect [ATE] adjustment: HR 4.08 [2.56-6.50], p<0.0001; patient-level: HR 2.63 [1.62-4.26], p<0.0001; 30-day ScT: lesion-level: HR 3.78 [1.51-9.44], p=0.005; patient-level: HR 3.61 [1.48-8.83], p=0.005) (Supplementary Table 2-Supplementary Table 5). The incidence of ScR before one year and that of TLR after one year was numerically, but not statistically, greater in the complex lesions group. There was no difference in the rate of ScT beyond one month after implantation. There was also no difference in the rate of all other events including death, cardiovascular death, myocardial infarction, and target vessel myocardial infarction.
THE ROLE OF IMPLANTATION TECHNIQUE
Post-dilatation alone had no effect on the rate of ScR (complex lesions group: p=0.6425; simple lesions: p=0.2089) or on that of ScT (complex: p=0.3478; simple p=0.3816) in either group. Post-procedural RVD was not a predictor of ScT in complex lesions (ScT: p=0.52, HR 1.27 [0.62-2.62]) but it was associated with ScR (p=0.0003, HR 0.43 [0.28-0.68]). Similar associations were observed in simple lesions (p=0.2203 and <0.0001). When the analysis was limited to vessels with an RVD comprising between 2.5 and 3.5 mm, complex lesions remained a predictor of ScT and ScR (p=0.0733, HR 3.1511 [0.9624-10.3171] for ScT; p<0.0001, HR 12.3185 [5.3165-28.5426], for ScR). The degree of post-procedural residual stenosis was a predictor of ScR and ScT in complex lesions (ScR: p<0.0001, HR 1.10 [1.07-1.14]; ScT: p=0.0021, HR 1.06 [1.02-1.09]) and in simple ones (ScR: p=0.0002, HR 1.07 [1.03-1.11]; ScT: p=0.0028, HR 1.06 [1.02-1.10]).
The combination of the implantation parameters described above (“optimal implantation technique”) was associated with a significant reduction in the rate of ScT both in patients with complex lesions (three-year incidence of ScT: from 9.5% to 2.3%, 83% reduction, p=0.0203, HR 0.17 [0.04-0.76]) as well as in those with simple lesions (three-year incidence: 6.1% vs. 0.5%, 92% reduction, p=0.0260, HR 0.097 [0.01-0.75]) (Figure 3). In patients in whom this “optimal implantation technique” was applied, the incidence of early ScT was not different between groups (0.8% vs. 0.5%, HR 1.3823 [0.0834-22.9206], p=0.8181).
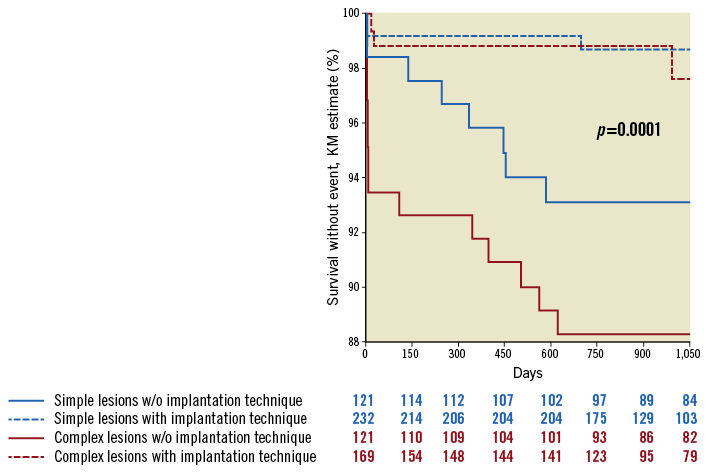
Figure 3. Application of an optimal implantation technique reduced the risk of ScT in both groups.
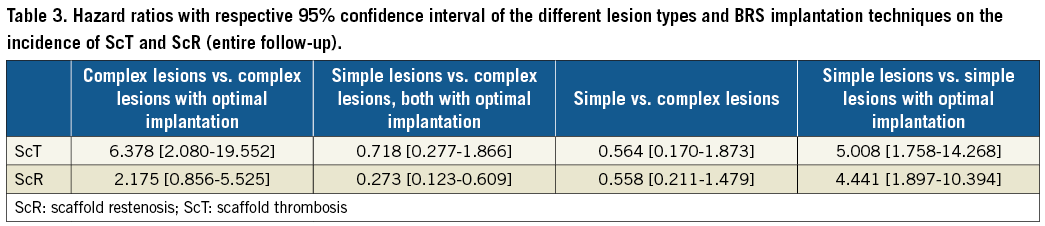
Figure 4 describes the impact of the implantation technique on the rates of ScR. As for ScT, the rate of ScR was lower in both simple and complex lesions (respectively, by 59% and 89%) when the optimal implantation technique was used. However, the lesion type remained a predictor of ScR (7.0% in complex vs. 1.6% in simple lesions, HR 5.11 [1.07-24.39], p=0.0420). The HRs associated with the different combinations of lesion complexity and implantation technique are presented in Table 3.
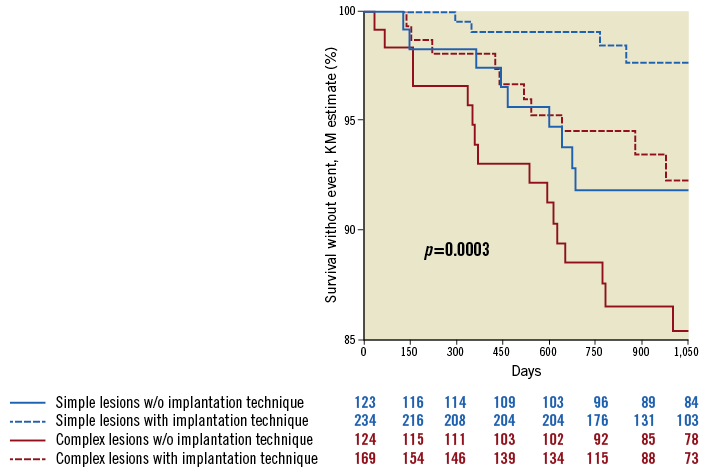
Figure 4. Application of an optimal implantation technique reduced the risk of ScR in both groups, but the risk remained higher in the complex lesions group.
Discussion
We report on the impact of lesion complexity on the outcome after BRS implantation in a series of consecutive patients. In this cohort, treatment of complex lesions with BRS was associated with a higher risk of early thrombosis and with in-scaffold restenosis. Of note, important differences in the clinical characteristics have to be acknowledged (patients in the simple lesions group were older, had worse renal function and a higher incidence of hypertension and prior stroke, while an acute presentation was more frequent in the complex group), but the impact of lesion complexity remained even after adjustment for these variables in a propensity score analysis.
Although the differences were numerically very small, treatment of complex lesions was associated with a higher rate of residual stenosis, which remained a predictor of both thrombosis and restenosis. As for the implantation parameters, post-dilatation alone had no impact, but event rates were reduced when a combination of factors (post-procedural RVD, sizing of the BRS, predilatation/post-dilatation) was respected. While the risk of ScT was completely offset by an optimised implantation, that of restenosis remained higher in complex vs. simple lesions, which possibly points to two different mechanisms for the two events. Taken together, these data demonstrate that the use of BRS is safe even in complex lesions, as long as the criteria recommended for an optimal implantation are respected and a slightly higher incidence of restenosis, compatible with DES data, is accepted17,18. Also, our data allow formulating the hypothesis that an adequate implantation may improve outcomes independently of lesion complexity15,19.
As in the general population, device and vessel expansion remain important parameters, especially in the early and vulnerable phase after revascularisation15. Confirming this concept, in a recent paper enrolling patients treated with BRS for complex lesions, intracoronary imaging performed at the time of implantation evidenced the need for further more aggressive post-dilatation in as many as ~50% of the lesions treated20. In addition, implantation of BRS in complex lesions was associated with a higher tissue prolapse area and greater incidence of malapposition at the proximal edge, whereas the use of predilatation balloons proportionally larger than those used for metallic stents was required to reach similar minimal and mean lumen areas. The experience with scoring devices or rotablator for the preparation of calcific lesions prior to BRS implantation remains small21.
Limitations
The observational nature of this registry, with an inherent bias with regard to patient/lesion selection, and the number of patients lost to follow-up (7% at three years) need to be acknowledged. As with any classification system, the ACC/AHA lesion classification provides important information but does not reflect the entire anatomical complexity of the lesions. In the absence of a more comprehensive system to stratify the risk associated with different lesion characteristics, however, the ACC/AHA classification continues to be the standard of reference. Also, the prevalence of ostial or bifurcation lesions with small side branches was lower in the complex lesions group, an apparently paradoxical finding probably due to the fact that metallic drug-eluting stents were systematically used for “true” bifurcation lesions or for ostial lesions with additional features of complexity (e.g., very calcific lesions). The fact that the final result (residual stenosis, ratio of MLD to nominal BRS) was assessed visually (QCA) without intracoronary imaging is also a limitation. Systematic use of imaging or functional techniques might improve the detection of suboptimal results and potentially patient prognosis. A hybrid stenting approach was used in many cases and significantly more frequently in patients with complex lesions, which makes the interpretation of some of the endpoints (death, device-oriented composite endpoint, any myocardial infarction) more complex. The significant difference in clinical presentation of the patient groups poses a potential selection bias. On the other hand, hybrid stenting and clinical presentation were accounted for in the propensity score analysis, which confirms the validity of our findings with regard to early ScT and ScR.
Although an external independent laboratory was not involved, QCA and OCT data were analysed using standard operating procedures by dedicated staff otherwise independent of the study.
Conclusions
The use of BRS in complex coronary lesions is safe but, as expected, is associated with higher event rates. The technique used at the time of the implantation offsets the risk of thrombosis and mitigates that of restenosis. The results of trials enrolling patients with high-risk lesions are awaited.
| Impact on daily practice Bioresorbable scaffolds were brought to the market without restriction as to the type of lesions and patients treated. The risk of early thrombosis and restenosis was reduced by ~80% when an optimal implantation technique was used. |
Acknowledgements
The authors are grateful to Markus Nagler for his assistance in performing the propensity score analysis.
Conflict of interest statement
T. Gori and T. Münzel have received speaker’s honoraria from Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Methods.
Supplementary Table 1. One-, 2-, and 3-year crude estimates based on the number of patients with follow-up available.
Supplementary Table 2. Distribution of risk factors after propensity score (PS) adjustment, scaffold thrombosis at 30 days, lesion-level analysis.
Supplementary Table 3. Distribution of risk factors after PS adjustment, in-scaffold restenosis (including diabetes), lesion level.
Supplementary Table 4. Distribution of risk factors after PS adjustment, in-scaffold thrombosis, patient level.
Supplementary Table 5. Distribution of risk factors after PS adjustment, in-scaffold restenosis (including diabetes), patient level.
To read the full content of this article, please download the PDF.

